Biosimilar erythropoietin in anemia treatment (BEAT)-Efficacy and safety of a 1:1 dose conversion from EPREX® to EPIAO® in patients with end-stage renal disease on hemodialysis: A prospective, randomized, double blind, parallel group study
- PMID: 36451454
- PMCID: PMC9704908
- DOI: 10.1097/MD.0000000000031426
Biosimilar erythropoietin in anemia treatment (BEAT)-Efficacy and safety of a 1:1 dose conversion from EPREX® to EPIAO® in patients with end-stage renal disease on hemodialysis: A prospective, randomized, double blind, parallel group study
Abstract
Background: EPREX®/ERYPO®/PROCRIT® (epoetin alfa, Janssen-Cilag GmbH) was the first available recombinant human erythropoietin (rHuEPO) and was universally reference product as per the recommendation provided by European Medicines Agency. EPIAO® is a biosimilar formulation of EPREX®, and making it a 1:1 dose conversion from EPREX® according to recommendation of European Medicines Agency. This study evaluated the clinical efficacy and safety of EPIAO® in subjects with end-stage renal disease receiving hemodialysis after intravenous administration.
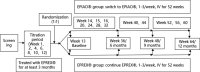
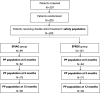
Methods: This study was a multicenter, prospective, randomized, double-blind, parallel-group, 2-cohort, maintenance phase, therapeutic equivalence study to evaluate a 1:1 dose conversion from EPREX® to EPIAO® in terms of clinical efficacy and safety that was conducted at 20 sites in 2 countries in patients with end-stage renal disease on hemodialysis. Eligible subjects were treated with EPREX® (reference product of epoetin) for a period of at least 3 months before the treatment period, and then were randomly assigned to the group of EPREX® or EPIAO®. Primary endpoints were mean absolute change in hemoglobin level and mean absolute change in weekly epoetin dosage from baseline to 6 months after treatment with EPIAO®/EPREX® in parallel groups.
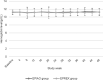
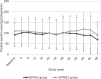
Results: A total of 200 people received the random intervention and were included in the safety set. After 6, 9, and 12 months of treatment with EPIAO® or EPREX®, there were no significant differences in the hemoglobin levels of the 2 groups compared with baseline. The 95% confidence interval for the treatment difference was within the predetermined acceptable range: ±0.5 g/dL. There were no significant differences in the epoetin dosage of the 2 groups compared with the baseline. The 95% confidence interval for the treatment difference was within the predetermined acceptable range: ± 45 IU/kg. There were no significant differences in the incidence of adverse events between the EPIAO® and EPREX® groups. Most adverse events were mild to moderate and were reverted/resolved.
Conclusion: EPIAO® demonstrated promising effectiveness and manageable safety in patients with end-stage renal disease on hemodialysis.
Trial registration: ClinicalTrials.gov NCT02947438.
Copyright © 2022 the Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
References
-
- Ammirati AL. Chronic kidney disease. Revista da Associacao Medica Brasileira. 2020;66 (Suppl 1):s03–s09. - PubMed
-
- Gafter-Gvili A, Schechter A, Rozen-Zvi B. Iron deficiency anemia in chronic kidney disease. Acta Haematol. 2019;142:44–50. - PubMed
-
- Atkinson MA, Warady BA. Anemia in chronic kidney disease. Pediatr Nephrol. 2018;33:227–38. - PubMed
-
- Salamin O, Kuuranne T, Saugy M, et al. . Erythropoietin as a performance-enhancing drug: its mechanistic basis, detection, and potential adverse effects. Mol Cell Endocrinol. 2018;464:75–87. - PubMed