Presentation and integration of multiple signals that modulate oligodendrocyte lineage progression and myelination
- PMID: 36451655
- PMCID: PMC9701731
- DOI: 10.3389/fncel.2022.1041853
Presentation and integration of multiple signals that modulate oligodendrocyte lineage progression and myelination
Abstract
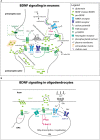
Myelination is critical for fast saltatory conduction of action potentials. Recent studies have revealed that myelin is not a static structure as previously considered but continues to be made and remodeled throughout adulthood in tune with the network requirement. Synthesis of new myelin requires turning on the switch in oligodendrocytes (OL) to initiate the myelination program that includes synthesis and transport of macromolecules needed for myelin production as well as the metabolic and other cellular functions needed to support this process. A significant amount of information is available regarding the individual intrinsic and extrinsic signals that promote OL commitment, expansion, terminal differentiation, and myelination. However, it is less clear how these signals are made available to OL lineage cells when needed, and how multiple signals are integrated to generate the correct amount of myelin that is needed in a given neural network state. Here we review the pleiotropic effects of some of the extracellular signals that affect myelination and discuss the cellular processes used by the source cells that contribute to the variation in the temporal and spatial availability of the signals, and how the recipient OL lineage cells might integrate the multiple signals presented to them in a manner dialed to the strength of the input.
Keywords: BDNF; Fyn; L1; NG2; OPC; neuron-glia communication; oligodendrocyte; trafficking.
Copyright © 2022 Fekete and Nishiyama.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Agresti C., Meomartini M. E., Amadio S., Ambrosini E., Volonté C., Aloisi F., et al. (2005). ATP regulates oligodendrocyte progenitor migration, proliferation and differentiation: involvement of metabotropic P2 receptors. Brain Res. Brain Res. Rev. 48, 157–165. 10.1016/j.brainresrev.2004.12.005 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

