Fermented Astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis
- PMID: 36451737
- PMCID: PMC9702530
- DOI: 10.3389/fnut.2022.1035912
Fermented Astragalus and its metabolites regulate inflammatory status and gut microbiota to repair intestinal barrier damage in dextran sulfate sodium-induced ulcerative colitis
Abstract
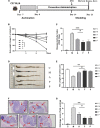
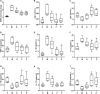
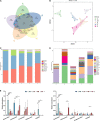
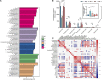
Fermentation represents an efficient biotechnological approach to increase the nutritional and functional potential of traditional Chinese medicine. In this study, Lactobacillus plantarum was used to ferment traditional Chinese medicine Astragalus, the differential metabolites in the fermented Astragalus (FA) were identified by ultra-performance liquid chromatography-Q Exactive hybrid quadrupole-Orbitrap mass spectrometry (UPLC-Q-Exactive-MS), and the ameliorating effect of FA on dextran sulfate sodium (DSS)-induced colitis in mice were further explored. The results showed that 11 differential metabolites such as raffinose, progesterone and uridine were identified in FA, which may help improve the ability of FA to alleviate colitis. Prophylactic FA supplementation effectively improved DAI score, colon length and histopathological lesion in DSS-treated mice. The abnormal activation of the intestinal immune barrier in mice was controlled after FA supplementation, the contents of myeloperoxidase (MPO) and IgE were reduced and the contents of IgA were increased. The intestinal pro-inflammatory factors TNF-α, IL-1β, IL-6, and IL-17 were down-regulated and the anti-inflammatory factors IL-10 and TGF-β were up-regulated, suggesting that FA can intervene in inflammatory status by regulating the balance of Th1/Th2/Th17/Treg related cytokines. In addition, FA supplementation modified the structure of the intestinal microbiota and enriched the abundance of Akkermansia and Alistipes, which were positively associated with the production of short-chain fatty acids. These microbes and their metabolites induced by FA also be involved in maintaining the intestinal mucosal barrier integrity by affecting mucosal immunity. We observed that intestinal tight junction protein and mucous secreting protein ZO-1, occludin, and MUC2 genes expression were more pronounced in mice supplemented with FA compared to unfermented Astragalus, along with modulation of intestinal epithelial cells (IECs) apoptosis, verifying the intestinal mucosal barrier repaired by FA. This study is the first to suggest that FA as a potential modulator can more effectively regulate the inflammatory status and gut microbiota to repair the intestinal barrier damage caused by colitis.
Keywords: Astragalus; fermentation; gut microbiota; inflammation; intestinal barrier.
Copyright © 2022 Li, Ma, Li, Wang, Huo, Lin, Li, Yang, Zhang, Yang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Bovine Lactoferrin Protects Dextran Sulfate Sodium Salt Mice Against Inflammation and Impairment of Colonic Epithelial Barrier by Regulating Gut Microbial Structure and Metabolites.Front Nutr. 2021 Apr 16;8:660598. doi: 10.3389/fnut.2021.660598. eCollection 2021. Front Nutr. 2021. PMID: 33954162 Free PMC article.
-
Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity.J Ethnopharmacol. 2023 Jan 10;300:115741. doi: 10.1016/j.jep.2022.115741. Epub 2022 Sep 24. J Ethnopharmacol. 2023. PMID: 36162543
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota.Biomed Pharmacother. 2021 May;137:111420. doi: 10.1016/j.biopha.2021.111420. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761623
-
Repairing the intestinal mucosal barrier of traditional Chinese medicine for ulcerative colitis: a review.Front Pharmacol. 2023 Oct 24;14:1273407. doi: 10.3389/fphar.2023.1273407. eCollection 2023. Front Pharmacol. 2023. PMID: 37942490 Free PMC article. Review.
-
Hydrogen Regulates Ulcerative Colitis by Affecting the Intestinal Redox Environment.J Inflamm Res. 2024 Feb 12;17:933-945. doi: 10.2147/JIR.S445152. eCollection 2024. J Inflamm Res. 2024. PMID: 38370464 Free PMC article. Review.
Cited by
-
Danggui Shaoyao San Alleviates Early Cognitive Impairment in Alzheimer's Disease Mice Through IRS1/GSK3β/Wnt3a-β-Catenin Pathway.Brain Behav. 2024 Oct;14(10):e70056. doi: 10.1002/brb3.70056. Brain Behav. 2024. PMID: 39344343 Free PMC article.
-
Role of traditional Chinese medicine in age-related macular degeneration: exploring the gut microbiota's influence.Front Pharmacol. 2024 Jan 25;15:1356324. doi: 10.3389/fphar.2024.1356324. eCollection 2024. Front Pharmacol. 2024. PMID: 38333011 Free PMC article. Review.
-
Fermentation: improvement of pharmacological effects and applications of botanical drugs.Front Pharmacol. 2024 Aug 26;15:1430238. doi: 10.3389/fphar.2024.1430238. eCollection 2024. Front Pharmacol. 2024. PMID: 39253373 Free PMC article. Review.
-
Research state of the herbal medicine Huangqi (Radix Astragali): A global and bibliometric study.Medicine (Baltimore). 2024 Feb 23;103(8):e37277. doi: 10.1097/MD.0000000000037277. Medicine (Baltimore). 2024. PMID: 38394541 Free PMC article.
-
Potential application mechanism of traditional Chinese medicine in treating immune checkpoint inhibitor-induced colitis.Front Immunol. 2024 Apr 10;15:1366489. doi: 10.3389/fimmu.2024.1366489. eCollection 2024. Front Immunol. 2024. PMID: 38660314 Free PMC article. Review.
References
-
- Zhang Y, Liu W, Zhang D, Yang Y, Wang X, Li L. Fermented and germinated processing improved the protective effects of foxtail millet whole grain against dextran sulfate sodium-induced acute ulcerative colitis and gut microbiota dysbiosis in C57bl/6 mice. Front Nutr. (2021) 8:694936. 10.3389/fnut.2021.694936 - DOI - PMC - PubMed
-
- Peters V, Bolte L, Schuttert E, Andreu-Sanchez S, Dijkstra G, Weersma R, et al. Western and carnivorous dietary patterns are associated with greater likelihood of ibd development in a large prospective population-based cohort. J Crohns Colitis. (2021) 16:jjab219. 10.1093/ecco-jcc/jjab219 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

