Extracellular vesicle-derived miRNAs improve stem cell-based therapeutic approaches in muscle wasting conditions
- PMID: 36451814
- PMCID: PMC9702803
- DOI: 10.3389/fimmu.2022.977617
Extracellular vesicle-derived miRNAs improve stem cell-based therapeutic approaches in muscle wasting conditions
Abstract
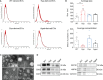
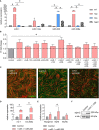
Skeletal muscle holds an intrinsic capability of growth and regeneration both in physiological conditions and in case of injury. Chronic muscle illnesses, generally caused by genetic and acquired factors, lead to deconditioning of the skeletal muscle structure and function, and are associated with a significant loss in muscle mass. At the same time, progressive muscle wasting is a hallmark of aging. Given the paracrine properties of myogenic stem cells, extracellular vesicle-derived signals have been studied for their potential implication in both the pathogenesis of degenerative neuromuscular diseases and as a possible therapeutic target. In this study, we screened the content of extracellular vesicles from animal models of muscle hypertrophy and muscle wasting associated with chronic disease and aging. Analysis of the transcriptome, protein cargo, and microRNAs (miRNAs) allowed us to identify a hypertrophic miRNA signature amenable for targeting muscle wasting, consisting of miR-1 and miR-208a. We tested this signature among others in vitro on mesoangioblasts (MABs), vessel-associated adult stem cells, and we observed an increase in the efficiency of myogenic differentiation. Furthermore, injections of miRNA-treated MABs in aged mice resulted in an improvement in skeletal muscle features, such as muscle weight, strength, cross-sectional area, and fibrosis compared to controls. Overall, we provide evidence that the extracellular vesicle-derived miRNA signature we identified enhances the myogenic potential of myogenic stem cells.
Keywords: aging; extracellular vesicle; hypertrophy; miRNA; muscular dystrophy; skeletal muscle.
Copyright © 2022 Yedigaryan, Martínez-Sarrà, Giacomazzi, Giarratana, van der Veer, Rotini, Querceto, Grosemans, Cortés-Calabuig, Salucci, Battistelli, Falcieri, Gijsbers, Quattrocelli, Peng Koh, De Waele, Buyse, Derua and Sampaolesi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

