Hydroxyurea ameliorates atherosclerosis in ApoE-/- mice by potentially modulating Niemann-Pick C1-like 1 protein through the gut microbiota
- PMID: 36451858
- PMCID: PMC9706578
- DOI: 10.7150/thno.76805
Hydroxyurea ameliorates atherosclerosis in ApoE-/- mice by potentially modulating Niemann-Pick C1-like 1 protein through the gut microbiota
Abstract
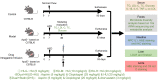
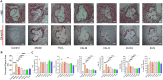
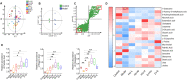
Rationale: The efficacy and mechanism of hydroxyurea in the treatment of atherosclerosis have rarely been reported. The goal of this study was to investigate the efficacy of hydroxyurea in high-fat diet-fed ApoE-/- mice against atherosclerosis and examine the possible mechanism underlying treatment outcomes. Methods: ApoE-/- mice were fed a high-fat diet for 1 month and then administered hydroxyurea by gavage continuously for 2 months. Aortic root hematoxylin-eosin (H&E) staining and oil red O staining were used to verify the efficacy of hydroxyurea; biochemical methods and ELISA were used to detect changes in relevant metabolites in serum. 16S rRNA was used to detect composition changes in the intestinal bacterial community of animals after treatment with hydroxyurea. Metabolomics methods were used to identify fecal metabolites and their changes. Immunohistochemical staining and ELISA were used for the localization and quantification of intestinal NPC1L1. Results: We showed that aortic root HE staining and oil red O staining determined the therapeutic efficacy of hydroxyurea in the treatment of atherosclerosis in high-fat diet-fed ApoE-/- mice. Serological tests verified the ability of hydroxyurea to lower total serum cholesterol and LDL cholesterol. The gut microbiota was significantly altered after HU treatment and was significantly different from that after antiplatelet and statin therapy. Meanwhile, a metabolomic study revealed that metabolites, including stearic acid, palmitic acid and cholesterol, were significantly enriched in mouse feces. Further histological and ELISAs verified that the protein responsible for intestinal absorption of cholesterol in mice, NPC1L1, was significantly reduced after hydroxyurea treatment. Conclusions: In high-fat diet-fed ApoE-/- mice, hydroxyurea effectively treated atherosclerosis, lowered serum cholesterol, modulated the gut microbiota at multiple levels and affected cholesterol absorption by reducing NPC1L1 in small intestinal epithelial cells.
Keywords: Atherosclerosis; Gut microbiota; Hydroxyurea; Lipid metabolism; NPC1L1.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of Atherosclerosis and the Potential to Reduce the Global Burden of Atherothrombotic Disease. Circ Res. 2016;118:535–46. - PubMed
-
- Falk E. Pathogenesis of Atherosclerosis. Journal of the American College of Cardiology. 2006;47:C7–12. - PubMed
-
- Jonsson AL, Bäckhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. - PubMed
-
- Byrne RA, Colleran R. Aspirin for secondary prevention of cardiovascular disease. The Lancet. 2020;395:1462–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

