Anthracycline-induced cardiotoxicity and senescence
- PMID: 36452034
- PMCID: PMC9701822
- DOI: 10.3389/fragi.2022.1058435
Anthracycline-induced cardiotoxicity and senescence
Abstract
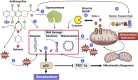
Cancer continues to place a heavy burden on healthcare systems around the world. Although cancer survivorship continues to improve, cardiotoxicity leading to cardiomyopathy and heart failure as a consequence of cancer therapy is rising, and yesterday's cancer survivors are fast becoming today's heart failure patients. Although the mechanisms driving cardiotoxicity are complex, cellular senescence is gaining attention as a major contributor to chemotherapy-induced cardiotoxicity and, therefore, may also represent a novel therapeutic target to prevent this disease. Cellular senescence is a well-recognized response to clinical doses of chemotherapies, including anthracyclines, and is defined by cell cycle exit, phenotypic alterations which include mitochondrial dysfunction, and the expression of the pro-senescent, pro-fibrotic, and pro-inflammatory senescence-associated phenotype. Senescence has an established involvement in promoting myocardial remodeling during aging, and studies have demonstrated that the elimination of senescence can attenuate the pathophysiology of several cardiovascular diseases. Most recently, pharmacology-mediated elimination of senescence, using a class of drugs termed senolytics, has been demonstrated to prevent myocardial dysfunction in preclinical models of chemotherapy-induced cardiotoxicity. In this review, we will discuss the evidence that anthracycline-induced senescence causes the long-term cardiotoxicity of anticancer chemotherapies, consider how the senescent phenotype may promote myocardial dysfunction, and examine the exciting possibility that targeting senescence may prove a therapeutic strategy to prevent or even reverse chemotherapy-induced cardiac dysfunction.
Keywords: anthracyclines; cancer; cardiac; chemotherapy; heart failure; senescence; senolytic.
Copyright © 2022 Booth, Redgrave, Folaranmi, Gill and Richardson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ale-Agha N., Jakobs P., Goy C., Zurek M., Rosen J., Dyballa-Rukes N., et al. (2021). Mitochondrial telomerase reverse transcriptase protects from myocardial ischemia/reperfusion injury by improving complex I composition and function. Circulation 144, 1876–1890. 10.1161/CIRCULATIONAHA.120.051923 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

