Enhanced pathogenicity by up-regulation of A20 after avian leukemia subgroup a virus infection
- PMID: 36452148
- PMCID: PMC9702354
- DOI: 10.3389/fvets.2022.1031480
Enhanced pathogenicity by up-regulation of A20 after avian leukemia subgroup a virus infection
Abstract
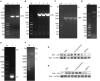
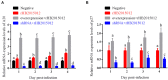
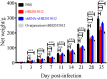
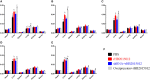
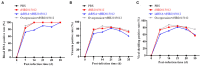
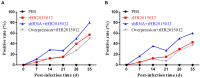
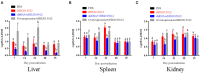
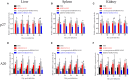
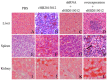
Avian leukemia virus subgroup A (ALV-A) infection slows chicken growth, immunosuppression, and tumor occurrence, causing economic loss to the poultry industry. According to previous findings, A20 has a dual role in promoting and inhibiting tumor formation but has rarely been studied in avians. In this study, A20 overexpression and shRNA interference recombinant adenoviruses were constructed and inoculated into chicken embryos, and ALV-A (rHB2015012) was inoculated into 1-day-old chicks. Analysis of body weight, organ index, detoxification, antibody production, organ toxin load, and Pathological observation revealed that A20 overexpression could enhance ALV-A pathogenicity. This study lays the foundation for subsequent exploration of the A20-mediated tumorigenic mechanism of ALV-A.
Keywords: A20; ALV-A; chicken; pathogenic; recombinant adenovirus.
Copyright © 2022 Chen, Wang, Yang, Fang, Liu, Liang and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
A20 Enhances the Expression of the Proto-Oncogene C-Myc by Downregulating TRAF6 Ubiquitination after ALV-A Infection.Viruses. 2022 Oct 7;14(10):2210. doi: 10.3390/v14102210. Viruses. 2022. PMID: 36298765 Free PMC article.
-
Synergistic pathogenesis of chicken infectious anemia virus and J subgroup of avian leukosis virus.Poult Sci. 2021 Nov;100(11):101468. doi: 10.1016/j.psj.2021.101468. Epub 2021 Sep 4. Poult Sci. 2021. PMID: 34624772 Free PMC article.
-
Synergistic pathogenic effects of co-infection of subgroup J avian leukosis virus and reticuloendotheliosis virus in broiler chickens.Avian Pathol. 2015;44(1):43-9. doi: 10.1080/03079457.2014.993359. Avian Pathol. 2015. PMID: 25484188
-
Recombinant invasive Lactobacillus plantarum expressing the J subgroup avian leukosis virus Gp85 protein induces protection against avian leukosis in chickens.Appl Microbiol Biotechnol. 2022 Jan;106(2):729-742. doi: 10.1007/s00253-021-11699-9. Epub 2021 Dec 31. Appl Microbiol Biotechnol. 2022. PMID: 34971411
-
Viral proliferation and expression of tumor-related gene in different chicken embryo fibroblasts infected with different tumorigenic phenotypes of avian leukosis virus subgroup J.Poult Sci. 2016 Oct 1;95(10):2383-90. doi: 10.3382/ps/pew180. Epub 2016 Jun 9. Poult Sci. 2016. PMID: 27609806
References
-
- Cui Z, Sun SH. Prevalence situation and prevention and control of avian leukosis. Chin J Vet Drug. (2009) 43:37–41.
-
- Relova D, Merino A. Avian leukosis virus subtype A and subtype J in different flocks with tumor disease. Rev Salud Anim. (2013) 35:70.
LinkOut - more resources
Full Text Sources

