3D cell culture based on artificial cells and hydrogel under microgravity for bottom-up microtissue constructs
- PMID: 36452208
- PMCID: PMC9704540
- DOI: 10.3389/fbioe.2022.1056652
3D cell culture based on artificial cells and hydrogel under microgravity for bottom-up microtissue constructs
Abstract
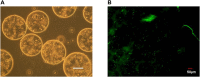
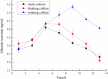
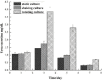
The use of hydrogel as a filling medium to recombine dispersed microencapsulated cells to form an embedded gel-cell microcapsule complex is a new idea based on bottom-up tissue construction, which is benefit for cell distribution and of great significance for tissue construction research in vitro. In this experiment, sodium alginate and chitosan were used as the main materials, rat normal liver cell BRL-3A was used as the model cell to prepare "artificial cells". Silkworm pupa was used as raw material to extract silk fibroin solution, which was prepared by ultrasound to be the silk fibroin gel; silk fibroin hydrogel-microencapsulated hepatocyte embedded complex was then prepared by using silk fibroin gel as filling medium; the complex was cultured under three modes (static, shaking, and 3D microgravity), and the tissue forming ability of rat hepatocytes was investigated. The results showed that the microgravity culture condition can enhance the cell proliferation and promote the formation of cell colonies in the microcapsules; silk fibroin can form an embedded gel-cell microcapsule complex with microencapsulated cells, which provided mechanical support for the structure of the composite. We hope that this bottom-up construction system will have potential applications in the fields of cell culture and tissue construction.
Keywords: artificial cell; bottom-up; hydrogel; microgravity culture; tissue engineering.
Copyright © 2022 Long, Shi, He, Tian, Wang and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Costantini D., Overi D., Casadei L., Cardinale V., Nevi L., Carpino G., et al. (2019). Simulated microgravity promotes the formation of tridimensional cultures and stimulates pluripotency and a glycolytic metabolism in human hepatic and biliary tree stem/progenitor cells. Sci. Rep. 9, 5559. 10.1038/s41598-019-41908-5 PubMed Abstract | 10.1038/s41598-019-41908-5 | Google Scholar - DOI - PMC - PubMed
-
- Farid E., Zahra H., Seyed M. T., Mojtaba D. (2018). Simulated microgravity condition alters the gene expression of some ECM and adhesion molecules in adipose derived stem cells. Int. J. Mol. Cell. Med. 7, 146–157. 10.22088/IJMCM.BUMS.7.3.146 PubMed Abstract | 10.22088/IJMCM.BUMS.7.3.146 | Google Scholar - DOI - PMC - PubMed
-
- Gomes J. M., Silva S. S., Fernandes E. M., Lobo F. C. M., Martín-Pastor M., Taboada P., et al. (2022). Silk fibroin/cholinium gallate-based architectures as therapeutic tools. Acta Biomater. 147, 168–184. 10.1016/j.actbio.2022.05.020 PubMed Abstract | 10.1016/j.actbio.2022.05.020 | Google Scholar - DOI - PubMed
-
- He L., Pan S., Li Y., Zhang L., Zhang W., Yi H., et al. (2016). Increased proliferation and adhesion properties of human dental pulp stem cells in PLGA scaffolds via simulated microgravity. Int. Endod. J. 49, 161–173. 10.1111/iej.12441 PubMed Abstract | 10.1111/iej.12441 | Google Scholar - DOI - PubMed
-
- Kundu B., Rajkhowa R., Kundu S. C., Wang X. (2013). Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 65, 457–470. 10.1016/j.addr.2012.09.043 PubMed Abstract | 10.1016/j.addr.2012.09.043 | Google Scholar - DOI - PubMed
LinkOut - more resources
Full Text Sources

