Development and validation of nomograms predicting postoperative survival in patients with chromophobe renal cell carcinoma
- PMID: 36452499
- PMCID: PMC9701834
- DOI: 10.3389/fonc.2022.982833
Development and validation of nomograms predicting postoperative survival in patients with chromophobe renal cell carcinoma
Abstract
Objective: The purpose of our study is to construct and validate nomograms that effectively predict postoperative overall survival and cancer-specific survival for patients with chromophobe renal cell carcinoma (chRCC).
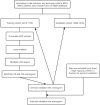
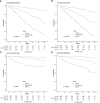
Method: Clinical, social, and pathological data from 6016 patients with chRCC collected from the SEER database were screened from 2004 to 2015. They were randomly assigned to a training cohort (n = 4212) and a validation cohort (n = 1804) at a 7:3 ratio. Cox regression and least absolute shrinkage and selection operator (LASSO) analyses were used to identify the prognostic factors affecting overall survival (OS) and cancer-specific survival (CSS) and establish nomograms. Their performance was validated internally and externally by calculating Harrell's C-indexes, area under the curve (AUC), calibration, and decision curves. For external validation, samples from postoperative patients with chRCC at 3 independent centers in Xuzhou, China, were collected. Risk stratification models were built according to the total scores of each patient. Kaplan-Meier curves were generated for the low-risk, intermediate-risk, and high-risk groups to evaluate survival.
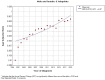
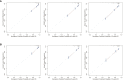
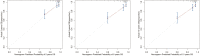
Results: The C-indexes, AUC curves, and decision curves revealed the high ability of the nomograms in predicting OS and CSS, overall better than that of AJCC and TNM staging. Moreover, in internal and external validation, the calibration curves of 5-, 8-, and 10-year OS agreed with the actual survival. Kaplan-Meier curves indicated significant differences in survival rates among the 3 risk groups in OS or CSS.
Conclusion: The nomograms showed favourable predictive power for OS and CSS. Thus, they should contribute to evaluating the prognosis of patients with chRCC. Furthermore, the risk stratification models established on the nomograms can guide the prognosis of patients and further treatment.
Keywords: SEER; cancer-specific survival; chromophobe renal cell carcinoma; nomogram; overall survival; prognosis; validation.
Copyright © 2022 Li, Zhu, He, Ashok, Xue, Liu, Ding and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
LinkOut - more resources
Full Text Sources

