Assessment of spatial transcriptomics for oncology discovery
- PMID: 36452860
- PMCID: PMC9701619
- DOI: 10.1016/j.crmeth.2022.100340
Assessment of spatial transcriptomics for oncology discovery
Abstract
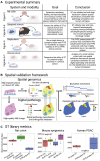
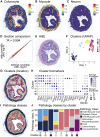
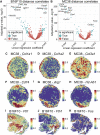
Tumor heterogeneity is a major challenge for oncology drug discovery and development. Understanding of the spatial tumor landscape is key to identifying new targets and impactful model systems. Here, we test the utility of spatial transcriptomics (ST) for oncology discovery by profiling 40 tissue sections and 80,024 capture spots across a diverse set of tissue types, sample formats, and RNA capture chemistries. We verify the accuracy and fidelity of ST by leveraging matched pathology analysis, which provides a ground truth for tissue section composition. We then use spatial data to demonstrate the capture of key tumor depth features, identifying hypoxia, necrosis, vasculature, and extracellular matrix variation. We also leverage spatial context to identify relative cell-type locations showing the anti-correlation of tumor and immune cells in syngeneic cancer models. Lastly, we demonstrate target identification approaches in clinical pancreatic adenocarcinoma samples, highlighting tumor intrinsic biomarkers and paracrine signaling.
Keywords: biomarkers; cancer biology; cancer genomics; digital pathology; genomics; oncology; pancreatic cancer; spatial genomics; spatial transcriptomics; tumors.
© 2022 Bristol-Myers Squibb.
Conflict of interest statement
A. Lyubetskaya, B.R., A.F., A. Lewin, I.N., E.P., R.G., S.K., A.F., K. Mosure, N.V.W., K. Mavrakis, K. MacIsaac, B.C., and E.D. are employees and shareholder of Bristol Myers Squibb.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

