Neutrophils Protect Against Staphylococcus aureus Endocarditis Progression Independent of Extracellular Trap Release
- PMID: 36453281
- PMCID: PMC9869964
- DOI: 10.1161/ATVBAHA.122.317800
Neutrophils Protect Against Staphylococcus aureus Endocarditis Progression Independent of Extracellular Trap Release
Abstract
Background: Infective endocarditis (IE) is characterized by an infected thrombus at the heart valves. How bacteria bypass the immune system and cause these thrombi remains unclear. Neutrophils releasing NETs (neutrophil extracellular traps) lie at this interface between host defense and coagulation. We aimed to determine the role of NETs in IE immunothrombosis.
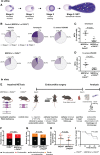
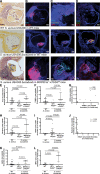
Methods: We used a murine model of Staphylococcus aureus endocarditis in which IE is provoked on inflamed heart valves and characterized IE thrombus content by immunostaining identifying NETs. Antibody-mediated neutrophil depletion and neutrophil-selective PAD4 (peptidylarginine deiminase 4)-knockout mice were used to clarify the role of neutrophils and NETs, respectively. S. aureus mutants deficient in key virulence factors related to immunothrombosis (nucleases or staphylocoagulases) were investigated.
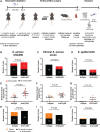
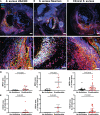
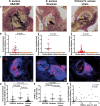
Results: Neutrophils releasing NETs were present in infected thrombi and within cellular infiltrates in the surrounding vasculature. Neutrophil depletion increased occurrence of IE, whereas neutrophil-selective impairment of NET formation did not alter IE occurrence. Absence of S. aureus nuclease, which degrades NETs, did not affect endocarditis outcome. In contrast, absence of staphylocoagulases (coagulase and von Willebrand factor binding protein) led to improved survival, decreased bacteremia, smaller infiltrates, and decreased tissue destruction. Significantly more NETs were present in these vegetations, which correlated with decreased bacteria and cell death in the adjacent vascular wall.
Conclusions: Neutrophils protect against IE independent of NET release. Absence of S. aureus coagulases, but not nucleases, reduced IE severity and increased NET levels. Staphylocoagulase-induced fibrin likely hampers NETs from constraining infection and the resultant tissue damage, a hallmark of valve destruction in IE.
Keywords: Staphylococcus aureus; coagulases; endocarditis; extracellular traps; neutrophils.
Figures



References
-
- Vogkou CT, Vlachogiannis NI, Palaiodimos L, Kousoulis AA. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. Eur J Clin Microbiol Infect Dis. 2016;35:1227–1245. doi: 10.1007/s10096-016-2660-6 - PubMed
-
- Yang X, Chen H, Zhang D, Shen L, An G, Zhao S. Global magnitude and temporal trend of infective endocarditis, 1990-2019: results from the global burden of disease study. Eur J Prev Cardiol. 2021;29:1227. doi: 10.1093/eurjpc/zwab184 - PubMed
-
- Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, et al. ; International Collaboration on Endocarditis Merged Database Study Group. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–514. doi: 10.1086/431979 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

