Translational bioengineering strategies for peripheral nerve regeneration: opportunities, challenges, and novel concepts
- PMID: 36453398
- PMCID: PMC9838159
- DOI: 10.4103/1673-5374.358616
Translational bioengineering strategies for peripheral nerve regeneration: opportunities, challenges, and novel concepts
Abstract
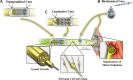
Peripheral nerve injuries remain a challenging problem in need of better treatment strategies. Despite best efforts at surgical reconstruction and postoperative rehabilitation, patients are often left with persistent, debilitating motor and sensory deficits. There are currently no therapeutic strategies proven to enhance the regenerative process in humans. A clinical need exists for the development of technologies to promote nerve regeneration and improve functional outcomes. Recent advances in the fields of tissue engineering and nanotechnology have enabled biomaterial scaffolds to modulate the host response to tissue repair through tailored mechanical, chemical, and conductive cues. New bioengineered approaches have enabled targeted, sustained delivery of protein therapeutics with the capacity to unlock the clinical potential of a myriad of neurotrophic growth factors that have demonstrated promise in enhancing regenerative outcomes. As such, further exploration of combinatory strategies leveraging these technological advances may offer a pathway towards clinically translatable solutions to advance the care of patients with peripheral nerve injuries. This review first presents the various emerging bioengineering strategies that can be applied for the management of nerve gap injuries. We cover the rationale and limitations for their use as an alternative to autografts, focusing on the approaches to increase the number of regenerating axons crossing the repair site, and facilitating their growth towards the distal stump. We also discuss the emerging growth factor-based therapeutic strategies designed to improve functional outcomes in a multimodal fashion, by accelerating axonal growth, improving the distal regenerative environment, and preventing end-organs atrophy.
Keywords: Schwann cells; bioengineering; biomaterials; growth hormone; insulin-like growth factor 1; nanotechnology; neurobiology; peripheral nerve regeneration; translational research.
Conflict of interest statement
None
Figures


References
-
- Adutler-Lieber S, Zaretsky I, Platzman I, Deeg J, Friedman N, Spatz JP, Geiger B. Engineering of synthetic cellular microenvironments:implications for immunity. J Autoimmun. 2014;54:100–111. - PubMed
-
- Ao Q. Progress of nerve bridges in the treatment of peripheral nerve disruptions. J Neurorestoratology. 2016;4:107–113.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

