Neuronal nitric oxide synthase/reactive oxygen species pathway is involved in apoptosis and pyroptosis in epilepsy
- PMID: 36453412
- PMCID: PMC9838157
- DOI: 10.4103/1673-5374.357906
Neuronal nitric oxide synthase/reactive oxygen species pathway is involved in apoptosis and pyroptosis in epilepsy
Abstract
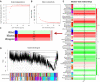
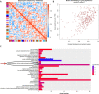
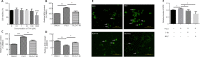
Dysfunction of neuronal nitric oxide synthase contributes to neurotoxicity, which triggers cell death in various neuropathological diseases, including epilepsy. Studies have shown that inhibition of neuronal nitric oxide synthase activity increases the epilepsy threshold, that is, has an anticonvulsant effect. However, the exact role and potential mechanism of neuronal nitric oxide synthase in seizures are still unclear. In this study, we performed RNA sequencing, functional enrichment analysis, and weighted gene coexpression network analysis of the hippocampus of tremor rats, a rat model of genetic epilepsy. We found damaged hippocampal mitochondria and abnormal succinate dehydrogenase level and Na+-K+-ATPase activity. In addition, we used a pilocarpine-induced N2a cell model to mimic epileptic injury. After application of neuronal nitric oxide synthase inhibitor 7-nitroindazole, changes in malondialdehyde, lactate dehydrogenase and superoxide dismutase, which are associated with oxidative stress, were reversed, and the increase in reactive oxygen species level was reversed by 7-nitroindazole or reactive oxygen species inhibitor N-acetylcysteine. Application of 7-nitroindazole or N-acetylcysteine downregulated the expression of caspase-3 and cytochrome c and reversed the apoptosis of epileptic cells. Furthermore, 7-nitroindazole or N-acetylcysteine downregulated the abnormally high expression of NLRP3, gasdermin-D, interleukin-1β and interleukin-18. This indicated that 7-nitroindazole and N-acetylcysteine each reversed epileptic cell death. Taken together, our findings suggest that the neuronal nitric oxide synthase/reactive oxygen species pathway is involved in pyroptosis of epileptic cells, and inhibiting neuronal nitric oxide synthase activity or its induced oxidative stress may play a neuroprotective role in epilepsy.
Keywords: RNA sequencing; Tremor rat; apoptosis; bioinformatics analysis; cell death; epilepsy; nitric oxide synthase; oxidative stress; pyroptosis; weighted gene co-expression network analysis.
Conflict of interest statement
None
Figures

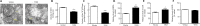

Similar articles
-
Inhibition of neuronal (type 1) nitric oxide synthase prevents hyperaemia and hippocampal lesions resulting from kainate-induced seizures.Neuroscience. 1998 Jun;84(3):791-800. doi: 10.1016/s0306-4522(97)00566-6. Neuroscience. 1998. PMID: 9579784
-
Tacrolimus protects hippocampal neurons of rats with status epilepticus through suppressing oxidative stress and inhibiting mitochondrial pathway of apoptosis.Brain Res. 2019 Jul 15;1715:176-181. doi: 10.1016/j.brainres.2019.02.031. Epub 2019 Mar 1. Brain Res. 2019. PMID: 30831086
-
Anticonvulsant effects of 7-nitroindazole in rodents with reflex epilepsy may result from L-arginine accumulation or a reduction in nitric oxide or L-citrulline formation.Br J Pharmacol. 1996 Sep;119(1):165-73. doi: 10.1111/j.1476-5381.1996.tb15690.x. Br J Pharmacol. 1996. PMID: 8872370 Free PMC article.
-
Mitochondrial dysfunction and oxidative stress in seizure-induced neuronal cell death.Acta Neurol Taiwan. 2010 Mar;19(1):3-15. Acta Neurol Taiwan. 2010. PMID: 20711885 Review.
-
Antioxidant Therapy Reduces Oxidative Stress, Restores Na,K-ATPase Function and Induces Neuroprotection in Rodent Models of Seizure and Epilepsy: A Systematic Review and Meta-Analysis.Antioxidants (Basel). 2023 Jul 7;12(7):1397. doi: 10.3390/antiox12071397. Antioxidants (Basel). 2023. PMID: 37507936 Free PMC article. Review.
Cited by
-
Pyroptosis in health and disease: mechanisms, regulation and clinical perspective.Signal Transduct Target Ther. 2024 Sep 20;9(1):245. doi: 10.1038/s41392-024-01958-2. Signal Transduct Target Ther. 2024. PMID: 39300122 Free PMC article. Review.
-
Rh-relaxin-2 attenuates oxidative stress and neuronal apoptosis via ERK-nNOS-NO pathway after germinal matrix hemorrhage in rats.Fluids Barriers CNS. 2025 Jan 15;22(1):8. doi: 10.1186/s12987-024-00616-7. Fluids Barriers CNS. 2025. PMID: 39815354 Free PMC article.
-
Genetic pathways in cerebral palsy: a review of the implications for precision diagnosis and understanding disease mechanisms.Neural Regen Res. 2024 Jul 1;19(7):1499-1508. doi: 10.4103/1673-5374.385855. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 38051892 Free PMC article.
-
Nanoemulsions of Cannabidiol, Δ9-Tetrahydrocannabinol, and Their Combination Similarly Exerted Anticonvulsant and Antioxidant Effects in Mice Treated with Pentyelenetetrazole.Pharmaceuticals (Basel). 2025 May 23;18(6):782. doi: 10.3390/ph18060782. Pharmaceuticals (Basel). 2025. PMID: 40573179 Free PMC article.
-
NLRP3 inflammasome and pyroptosis: implications in inflammation and multisystem disorders.PeerJ. 2025 Aug 15;13:e19887. doi: 10.7717/peerj.19887. eCollection 2025. PeerJ. 2025. PMID: 40832584 Free PMC article. Review.
References
-
- Abdel-Salam OME, Youness ER, Mohammed NA, Yassen NN, Khadrawy YA, El-Toukhy SE, Sleem AA. Nitric oxide synthase inhibitors protect against brain and liver damage caused by acute malathion intoxication. Asian Pac J Trop Med. 2017;10:773–786. - PubMed
-
- Akyuz E, Polat AK, Eroglu E, Kullu I, Angelopoulou E, Paudel YN. Revisiting the role of neurotransmitters in epilepsy:An updated review. Life Sci. 2021;265:118826. - PubMed
-
- Anaeigoudari A, Shafei MN, Soukhtanloo M, Sadeghnia HR, Reisi P, Beheshti F, Mohebbati R, Mousavi SM, Hosseini M. Lipopolysaccharide-induced memory impairment in rats is preventable using 7-nitroindazole. Arq Neuropsiquiatr. 2015;73:784–790. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

