Withaferin A inhibits ferroptosis and protects against intracerebral hemorrhage
- PMID: 36453416
- PMCID: PMC9838153
- DOI: 10.4103/1673-5374.355822
Withaferin A inhibits ferroptosis and protects against intracerebral hemorrhage
Abstract
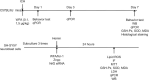
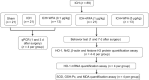
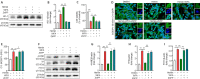
Recent studies have indicated that suppressing oxidative stress and ferroptosis can considerably improve the prognosis of intracerebral hemorrhage (ICH). Withaferin A (WFA), a natural compound, exhibits a positive effect on a number of neurological diseases. However, the effects of WFA on oxidative stress and ferroptosis-mediated signaling pathways to ICH remain unknown. In this study, we investigated the neuroprotective effects and underlying mechanism for WFA in the regulation of ICH-induced oxidative stress and ferroptosis. We established a mouse model of ICH by injection of autologous tail artery blood into the caudate nucleus and an in vitro cell model of hemin-induced ICH. WFA was injected intracerebroventricularly at 0.1, 1 or 5 µg/kg once daily for 7 days, starting immediately after ICH operation. WFA markedly reduced brain tissue injury and iron deposition and improved neurological function in a dose-dependent manner 7 days after cerebral hemorrhage. Through in vitro experiments, cell viability test showed that WFA protected SH-SY5Y neuronal cells against hemin-induced cell injury. Enzyme-linked immunosorbent assays in vitro and in vivo showed that WFA markedly decreased the level of malondialdehyde, an oxidative stress marker, and increased the activities of anti-oxidative stress markers superoxide dismutase and glutathione peroxidase after ICH. Western blot assay, quantitative polymerase chain reaction and immunofluorescence results demonstrated that WFA activated the nuclear factor E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling axis, promoted translocation of Nrf2 from the cytoplasm to nucleus, and increased HO-1 expression. Silencing Nrf2 with siRNA completely reversed HO-1 expression, oxidative stress and protective effects of WFA. Furthermore, WFA reduced hemin-induced ferroptosis. However, after treatment with an HO-1 inhibitor, the neuroprotective effects of WFA against hemin-induced ferroptosis were weakened. MTT test results showed that WFA combined with ferrostatin-1 reduced hemin-induced SH-SY5Y neuronal cell injury. Our findings reveal that WFA treatment alleviated ICH injury-induced ferroptosis and oxidative stress through activating the Nrf2/HO-1 pathway, which may highlight a potential role of WFA for the treatment of ICH.
Keywords: behavior; brain injuries; ferroptosis; heme oxygenase-1; hemorrhagic stroke; neuroprotection; nuclear factor E2-related factor 2; nuclear translocator; oxidative stress; stroke.
Conflict of interest statement
None
Figures
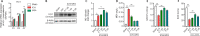





References
-
- Caso V, Mosconi MG. Lessons to be learned in intracerebral haemorrhage research. Lancet Neurol. 2021;20:779–780. - PubMed
-
- Chao OY, Pum ME, Li JS, Huston JP. The grid-walking test:assessment of sensorimotor deficits after moderate or severe dopamine depletion by 6-hydroxydopamine lesions in the dorsal striatum and medial forebrain bundle. Neuroscience. 2012;202:318–325. - PubMed
LinkOut - more resources
Full Text Sources

