Cyclin G1 induces maladaptive proximal tubule cell dedifferentiation and renal fibrosis through CDK5 activation
- PMID: 36453545
- PMCID: PMC9711881
- DOI: 10.1172/JCI158096
Cyclin G1 induces maladaptive proximal tubule cell dedifferentiation and renal fibrosis through CDK5 activation
Abstract
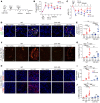
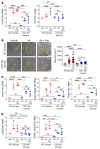
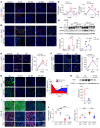
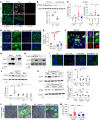
Acute kidney injury (AKI) occurs in approximately 13% of hospitalized patients and predisposes patients to chronic kidney disease (CKD) through the AKI-to-CKD transition. Studies from our laboratory and others have demonstrated that maladaptive repair of proximal tubule cells (PTCs), including induction of dedifferentiation, G2/M cell cycle arrest, senescence, and profibrotic cytokine secretion, is a key process promoting AKI-to-CKD transition, kidney fibrosis, and CKD progression. The molecular mechanisms governing maladaptive repair and the relative contribution of dedifferentiation, G2/M arrest, and senescence to CKD remain to be resolved. We identified cyclin G1 (CG1) as a factor upregulated in chronically injured and maladaptively repaired PTCs. We demonstrated that global deletion of CG1 inhibits G2/M arrest and fibrosis. Pharmacological induction of G2/M arrest in CG1-knockout mice, however, did not fully reverse the antifibrotic phenotype. Knockout of CG1 did not alter dedifferentiation and proliferation in the adaptive repair response following AKI. Instead, CG1 specifically promoted the prolonged dedifferentiation of kidney tubule epithelial cells observed in CKD. Mechanistically, CG1 promotes dedifferentiation through activation of cyclin-dependent kinase 5 (CDK5). Deletion of CDK5 in kidney tubule cells did not prevent G2/M arrest but did inhibit dedifferentiation and fibrosis. Thus, CG1 and CDK5 represent a unique pathway that regulates maladaptive, but not adaptive, dedifferentiation, suggesting they could be therapeutic targets for CKD.
Keywords: Cell cycle; Chronic kidney disease; Fibrosis; Nephrology.
Figures

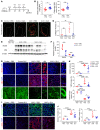
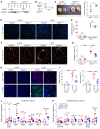
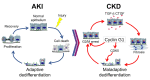
Comment in
- Decoupling dedifferentiation and G2-M arrest in kidney fibrosis doi: 10.1172/JCI163846
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK095072/DK/NIDDK NIH HHS/United States
- R01 DK069921/DK/NIDDK NIH HHS/United States
- UC2 DK126006/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- R35 HL139424/HL/NHLBI NIH HHS/United States
- I01 BX002025/BX/BLRD VA/United States
- R01 DK119212/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- R01 DK127589/DK/NIDDK NIH HHS/United States
- P30 DK114809/DK/NIDDK NIH HHS/United States
- R01 DK088327/DK/NIDDK NIH HHS/United States
- U01 DK127553/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- IK6 BX005240/BX/BLRD VA/United States
- I01 BX002196/BX/BLRD VA/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- R01 DK121101/DK/NIDDK NIH HHS/United States
- R01 DK117914/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States

