Lipo-Chitooligosaccharides Induce Specialized Fungal Metabolite Profiles That Modulate Bacterial Growth
- PMID: 36453934
- PMCID: PMC9764981
- DOI: 10.1128/msystems.01052-22
Lipo-Chitooligosaccharides Induce Specialized Fungal Metabolite Profiles That Modulate Bacterial Growth
Abstract
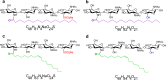
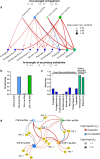
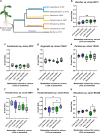
Lipo-chitooligosaccharides (LCOs) are historically known for their role as microbial-derived signaling molecules that shape plant symbiosis with beneficial rhizobia or mycorrhizal fungi. Recent studies showing that LCOs are widespread across the fungal kingdom have raised questions about the ecological function of these compounds in organisms that do not form symbiotic relationships with plants. To elucidate the ecological function of these compounds, we investigate the metabolomic response of the ubiquitous human pathogen Aspergillus fumigatus to LCOs. Our metabolomics data revealed that exogenous application of various types of LCOs to A. fumigatus resulted in significant shifts in the fungal metabolic profile, with marked changes in the production of specialized metabolites known to mediate ecological interactions. Using network analyses, we identify specific types of LCOs with the most significant effect on the abundance of known metabolites. Extracts of several LCO-induced metabolic profiles significantly impact the growth rates of diverse bacterial species. These findings suggest that LCOs may play an important role in the competitive dynamics of non-plant-symbiotic fungi and bacteria. This study identifies specific metabolomic profiles induced by these ubiquitously produced chemicals and creates a foundation for future studies into the potential roles of LCOs as modulators of interkingdom competition. IMPORTANCE The activation of silent biosynthetic gene clusters (BGC) for the identification and characterization of novel fungal secondary metabolites is a perpetual motion in natural product discoveries. Here, we demonstrated that one of the best-studied symbiosis signaling compounds, lipo-chitooligosaccharides (LCOs), play a role in activating some of these BGCs, resulting in the production of known, putative, and unknown metabolites with biological activities. This collection of metabolites induced by LCOs differentially modulate bacterial growth, while the LCO standards do not convey the same effect. These findings create a paradigm shift showing that LCOs have a more prominent role outside of host recognition of symbiotic microbes. Importantly, our work demonstrates that fungi use LCOs to produce a variety of metabolites with biological activity, which can be a potential source of bio-stimulants, pesticides, or pharmaceuticals.
Keywords: Aspergillus; bacteria; biosynthetic gene clusters; lipo-chitooligosaccharides; network analyses; secondary metabolites; synergism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Rush TA, Puech-Pagès V, Bascaules A, Jargeat P, Maillet F, Haouy A, Maës AQ, Carriel CC, Khokhani D, Keller-Pearson M, Tannous J, Cope KR, Garcia K, Maeda J, Johnson C, Kleven B, Choudhury QJ, Labbé J, Swift C, O’Malley MA, Bok JW, Cottaz S, Fort S, Poinsot V, Sussman MR, Lefort C, Nett J, Keller NP, Bécard G, Ané J-M. 2020. Lipo-chitooligosaccharides as regulatory signals of fungal growth and development. Nat Commun 11:3897. doi: 10.1038/s41467-020-17615-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
