Rationale for Use of Sphingosine-1-Phosphate Receptor Modulators in COVID-19 Patients: Overview of Scientific Evidence
- PMID: 36454249
- PMCID: PMC10282791
- DOI: 10.1089/jir.2022.0078
Rationale for Use of Sphingosine-1-Phosphate Receptor Modulators in COVID-19 Patients: Overview of Scientific Evidence
Abstract
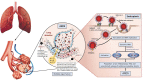
Maladjusted immune responses to the coronavirus disease 2019 (COVID-19), for example, cytokine release syndrome, may result in immunopathology and acute respiratory distress syndrome. Sphingosine-1-phosphate (S1P), a bioactive lipid mediator, and its S1P receptor (S1PR) are crucial in maintaining endothelial cell chemotaxis and barrier integrity. Apart from the S1P1 receptor-mediated mechanisms of sequestration of cytotoxic lymphocytes, including Th-17 and S1P1/2/3-mediated endothelial barrier functions, S1PR modulators may also attenuate cytokine release via activation of serine/threonine protein phosphatase 2A and enhance the pulmonary endothelial barrier via the c-Abl tyrosine kinase pathway. Chronic treatment with fingolimod (S1PR1,3,4,5 modulator) and siponimod (S1PR1,5 modulator) has demonstrated efficacy in reducing inflammatory disease activity and slowing down disease progression in multiple sclerosis. The decision to selectively suppress the immunity of a critically ill patient with COVID-19 remains a difficult choice. It has been suggested that treatment with fingolimod or siponimod may be appropriate to attenuate severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)-induced hyperinflammation in patients with COVID-19 since these patients are already monitored in an intensive care setting. Here, we review the use of S1PR modulators, fingolimod and siponimod, in regulating the inflammatory response to SARS-CoV-2 with the aim of understanding their potential rationale use in patients with COVID-19.
Keywords: cytokine suppression; fingolimod; immune response; siponimod.
Conflict of interest statement
T.H., G.G., and D.P.M. are full-time employees of Novartis Pharma AG, Basel, Switzerland. K.S.N., M.B., and O.P. are full-time employees of Novartis Institutes for Biomedical Research, Basel, Switzerland. M.d.M. and R.T. are full-time employees of Novartis Farma S.p.A., Origgio, Italy. V.B. and F.N. have no declaration of interest to declare. F.D. is an ex-employee of Novartis Pharma AG, Basel, Switzerland.
Figures



References
-
- Bergmann CC, Silverman RH. 2020. COVID-19: coronavirus replication, pathogenesis, and therapeutic strategies. Cleve Clin J Med 87(6):321–327. - PubMed
-
- Bigaud M, Dincer Z, Bollbuck B, Dawson J, Beckmann N, Beerli C, Fishli-Cavelti G, Nahler M, Angst D, Janser P, Otto H, Rosner E, Hersperger R, Bruns C, Quancard J. 2016. Pathophysiological consequences of a break in S1P1-dependent homeostasis of vascular permeability revealed by S1P1 competitive antagonism. PLoS One 11(12):e0168252. - PMC - PubMed
-
- Boro M, Balaji KN. 2017. CXCL1 and CXCL2 regulate NLRP3 inflammasome activation via G-protein–coupled receptor CXCR2. J Immunol 199(5):1660–1671. - PubMed
-
- Brinkmann V. 2007. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther 115(1):84–105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
