Human and rat microsomal metabolites of N-tert-butoxycarbonylmethamphetamine and its urinary metabolites in rat
- PMID: 36454489
- PMCID: PMC9715518
- DOI: 10.1007/s11419-021-00595-6
Human and rat microsomal metabolites of N-tert-butoxycarbonylmethamphetamine and its urinary metabolites in rat
Abstract
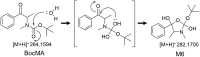
Purpose: N-tert-Butoxycarbonylmethamphetamine (BocMA), a masked derivative of methamphetamine (MA), converts into MA under acidic condition and potentially acts as a precursor to MA following ingestion. To investigate the metabolism and excretion of BocMA, metabolism tests were conducted using human liver microsomes (HLM), rat liver microsomes (RLM) and rat.
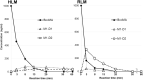
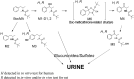
Methods: BocMA metabolites were analyzed after 1000-ng/mL BocMA incubation with microsomes for 3, 8, 13, 20, 30, and 60 min. Rats were administered intraperitoneal injections (20 mg/kg) of BocMA and their urine was collected in intervals for 72 h. Metabolites were detected by liquid chromatography-tandem mass spectrometry with five authentic standards.
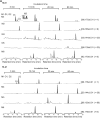
Results: Several metabolites including 4-hydroxy-BocMA, N-tert-butoxycarbonylephedrine and N-tert-butoxycarbonyl-cathinone were detected for HLM and RLM. In the administration test, three glucuronides of hydroxylated metabolites were detected. The total recovery values of BocMA and the metabolites during the first 72 h accounted for only 0.3% of the administered dose. Throughout the microsomal and administration experiments, MAs were not detected.
Conclusion: Hydroxylation, carbonylation and N-demethylation were proposed as metabolic pathways. However, BocMA and phase I metabolites were hardly detected in urine. This study provides useful information to interpret the possibility of BocMA intake as the cause of MA detection in biological sample.
Keywords: LC–MS/MS; Metabolite; Methamphetamine; Microsomes; N-tert-Butoxycarbonylmethamphetamine; Rat.
© 2021. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



References
-
- Stoneberg DM, Shukla RK, Global MMB. Methamphetamine trends: an evolving problem. Int Crim Justice Rev. 2018;28:136–161. doi: 10.1177/1057567717730104. - DOI
-
- Sunlive (2017) T-boc meth intercepted at border. http://sunlive.co.nz/news/149476-tboc-meth-intercepted-at-border.html. Accessed 16 Jul 2021
-
- Agami C, Couty F. The reactivity of the N-Boc protecting group: an underrated feature. Tetrahedron. 2002;58:2701–2724. doi: 10.1016/S0040-4020(02)00131-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

