Association of Initial SARS-CoV-2 Test Positivity With Patient-Reported Well-being 3 Months After a Symptomatic Illness
- PMID: 36454572
- PMCID: PMC9716377
- DOI: 10.1001/jamanetworkopen.2022.44486
Association of Initial SARS-CoV-2 Test Positivity With Patient-Reported Well-being 3 Months After a Symptomatic Illness
Abstract
Importance: Long-term sequelae after symptomatic SARS-CoV-2 infection may impact well-being, yet existing data primarily focus on discrete symptoms and/or health care use.
Objective: To compare patient-reported outcomes of physical, mental, and social well-being among adults with symptomatic illness who received a positive vs negative test result for SARS-CoV-2 infection.
Design, setting, and participants: This cohort study was a planned interim analysis of an ongoing multicenter prospective longitudinal registry study (the Innovative Support for Patients With SARS-CoV-2 Infections Registry [INSPIRE]). Participants were enrolled from December 11, 2020, to September 10, 2021, and comprised adults (aged ≥18 years) with acute symptoms suggestive of SARS-CoV-2 infection at the time of receipt of a SARS-CoV-2 test approved by the US Food and Drug Administration. The analysis included the first 1000 participants who completed baseline and 3-month follow-up surveys consisting of questions from the 29-item Patient-Reported Outcomes Measurement Information System (PROMIS-29; 7 subscales, including physical function, anxiety, depression, fatigue, social participation, sleep disturbance, and pain interference) and the PROMIS Short Form-Cognitive Function 8a scale, for which population-normed T scores were reported.
Exposures: SARS-CoV-2 status (positive or negative test result) at enrollment.
Main outcomes and measures: Mean PROMIS scores for participants with positive COVID-19 tests vs negative COVID-19 tests were compared descriptively and using multivariable regression analysis.
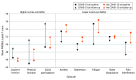
Results: Among 1000 participants, 722 (72.2%) received a positive COVID-19 result and 278 (27.8%) received a negative result; 406 of 998 participants (40.7%) were aged 18 to 34 years, 644 of 972 (66.3%) were female, 833 of 984 (84.7%) were non-Hispanic, and 685 of 974 (70.3%) were White. A total of 282 of 712 participants (39.6%) in the COVID-19-positive group and 147 of 275 participants (53.5%) in the COVID-19-negative group reported persistently poor physical, mental, or social well-being at 3-month follow-up. After adjustment, improvements in well-being were statistically and clinically greater for participants in the COVID-19-positive group vs the COVID-19-negative group only for social participation (β = 3.32; 95% CI, 1.84-4.80; P < .001); changes in other well-being domains were not clinically different between groups. Improvements in well-being in the COVID-19-positive group were concentrated among participants aged 18 to 34 years (eg, social participation: β = 3.90; 95% CI, 1.75-6.05; P < .001) and those who presented for COVID-19 testing in an ambulatory setting (eg, social participation: β = 4.16; 95% CI, 2.12-6.20; P < .001).
Conclusions and relevance: In this study, participants in both the COVID-19-positive and COVID-19-negative groups reported persistently poor physical, mental, or social well-being at 3-month follow-up. Although some individuals had clinically meaningful improvements over time, many reported moderate to severe impairments in well-being 3 months later. These results highlight the importance of including a control group of participants with negative COVID-19 results for comparison when examining the sequelae of COVID-19.
Conflict of interest statement
Figures

References
-
- Hernandez-Romieu AC, Carton TW, Saydah S, et al. . Prevalence of select new symptoms and conditions among persons aged younger than 20 years and 20 years or older at 31 to 150 days after testing positive or negative for SARS-CoV-2. JAMA Netw Open. 2022;5(2):e2147053. doi:10.1001/jamanetworkopen.2021.47053 - DOI - PMC - PubMed
-
- Hernandez-Romieu AC, Leung S, Mbanya A, et al. . Health care utilization and clinical characteristics of nonhospitalized adults in an integrated health care system 28-180 days after COVID-19 diagnosis—Georgia, May 2020–March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(17):644-650. doi:10.15585/mmwr.mm7017e3 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

