Comparing Acute IOP-Induced Lamina Cribrosa Deformations Premortem and Postmortem
- PMID: 36454578
- PMCID: PMC9728494
- DOI: 10.1167/tvst.11.12.1
Comparing Acute IOP-Induced Lamina Cribrosa Deformations Premortem and Postmortem
Abstract
Purpose: Lamina cribrosa (LC) deformations caused by elevated intraocular pressure (IOP) are believed to contribute to glaucomatous neuropathy and have therefore been extensively studied, in many conditions, from in vivo to ex vivo. We compare acute IOP-induced global and local LC deformations immediately before (premortem) and after (postmortem) sacrifice by exsanguination.
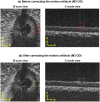
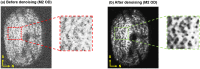
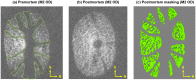
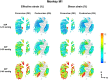
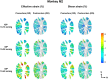
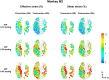
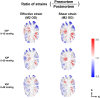
Methods: The optic nerve heads of three healthy monkeys 12 to 15 years old were imaged with spectral-domain optical coherence tomography under controlled IOP premortem and postmortem. Volume scans were acquired at baseline IOP (8-10 mm Hg) and at 15, 30, and 40 mm Hg IOP. A digital volume correlation technique was used to determine the IOP-induced three-dimensional LC deformations (strains) in regions visible premortem and postmortem.
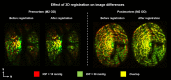
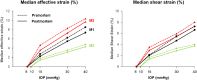
Results: Both conditions exhibited similar nonlinear relationships between IOP increases and LC deformations. Median effective and shear strains were, on average, over all eyes and pressures, smaller postmortem than premortem, by 14% and 11%, respectively (P's < 0.001). Locally, however, the differences in LC deformation between conditions were variable. Some regions were subjected premortem to triple the strains observed postmortem, and others suffered smaller deformations premortem than postmortem.
Conclusions: Increasing IOP acutely caused nonlinear LC deformations with an overall smaller effect postmortem than premortem. Locally, deformations premortem and postmortem were sometimes substantially different. We suggest that the differences may be due to weakened mechanical support from the unpressurized central retinal vessels postmortem.
Translational relevance: Additional to the important premortem information, comparison with postmortem provides a unique context essential to understand the translational relevance of all postmortem biomechanics literature.
Conflict of interest statement
Disclosure:
Figures




References
-
- Quigley HA. Glaucoma. Lancet. 2011; 377: 1367–1377. - PubMed
-
- Sigal IA. Interactions between geometry and mechanical properties on the optic nerve head. Invest Ophthalmol Vis Sci. 2009; 50: 2785–2795. - PubMed
-
- Quigley HA. Reappraisal of the mechanisms of glaucomatous optic nerve damage. Eye. 1987; 1: 318–322. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

