The PRK/Rubisco shunt strongly influences Arabidopsis seed metabolism and oil accumulation, affecting more than carbon recycling
- PMID: 36454674
- PMCID: PMC9940875
- DOI: 10.1093/plcell/koac338
The PRK/Rubisco shunt strongly influences Arabidopsis seed metabolism and oil accumulation, affecting more than carbon recycling
Abstract
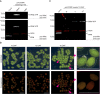
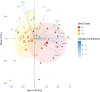
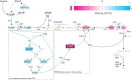
The carbon efficiency of storage lipid biosynthesis from imported sucrose in green Brassicaceae seeds is proposed to be enhanced by the PRK/Rubisco shunt, in which ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) acts outside the context of the Calvin-Benson-Bassham cycle to recycle CO2 molecules released during fatty acid synthesis. This pathway utilizes metabolites generated by the nonoxidative steps of the pentose phosphate pathway. Photosynthesis provides energy for reactions such as the phosphorylation of ribulose 5-phosphate by phosphoribulokinase (PRK). Here, we show that loss of PRK in Arabidopsis thaliana (Arabidopsis) blocks photoautotrophic growth and is seedling-lethal. However, seeds containing prk embryos develop normally, allowing us to use genetics to assess the importance of the PRK/Rubisco shunt. Compared with nonmutant siblings, prk embryos produce one-third less lipids-a greater reduction than expected from simply blocking the proposed PRK/Rubisco shunt. However, developing prk seeds are also chlorotic and have elevated starch contents compared with their siblings, indicative of secondary effects. Overexpressing PRK did not increase embryo lipid content, but metabolite profiling suggested that Rubisco activity becomes limiting. Overall, our findings show that the PRK/Rubisco shunt is tightly integrated into the carbon metabolism of green Arabidopsis seeds, and that its manipulation affects seed glycolysis, starch metabolism, and photosynthesis.
© The Author(s) 2022. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




References
-
- Andersson I, Backlund A (2008) Structure and function of Rubisco. Plant Physiol Biochem 46(3): 275–291 - PubMed
-
- Andriotis VM, Pike MJ, Kular B, Rawsthorne S, Smith AM (2010) Starch turnover in developing oilseed embryos. New Phytol 187(3): 791–804 - PubMed
-
- Arrivault S, Guenther M, Ivakov A, Feil R, Vosloh D, Van Dongen JT, Sulpice R, Stitt M (2009) Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. Plant J 59(5): 826–839 - PubMed
-
- Asokanthan PS, Johnson RW, Griffith M, Krol M (1997) The photosynthetic potential of canola embryos. Physiol Plant 101(2): 353–360
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

