CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond
- PMID: 36454715
- PMCID: PMC9988538
- DOI: 10.1152/physrev.00059.2021
CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond
Abstract
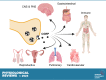
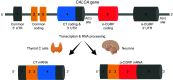
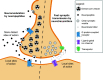
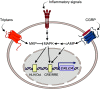
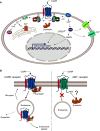
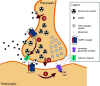
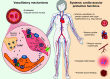
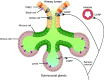
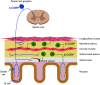
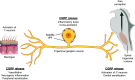
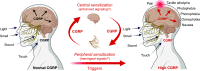
Calcitonin gene-related peptide (CGRP) is a neuropeptide with diverse physiological functions. Its two isoforms (α and β) are widely expressed throughout the body in sensory neurons as well as in other cell types, such as motor neurons and neuroendocrine cells. CGRP acts via at least two G protein-coupled receptors that form unusual complexes with receptor activity-modifying proteins. These are the CGRP receptor and the AMY1 receptor; in rodents, additional receptors come into play. Although CGRP is known to produce many effects, the precise molecular identity of the receptor(s) that mediates CGRP effects is seldom clear. Despite the many enigmas still in CGRP biology, therapeutics that target the CGRP axis to treat or prevent migraine are a bench-to-bedside success story. This review provides a contextual background on the regulation and sites of CGRP expression and CGRP receptor pharmacology. The physiological actions of CGRP in the nervous system are discussed, along with updates on CGRP actions in the cardiovascular, pulmonary, gastrointestinal, immune, hematopoietic, and reproductive systems and metabolic effects of CGRP in muscle and adipose tissues. We cover how CGRP in these systems is associated with disease states, most notably migraine. In this context, we discuss how CGRP actions in both the peripheral and central nervous systems provide a basis for therapeutic targeting of CGRP in migraine. Finally, we highlight potentially fertile ground for the development of additional therapeutics and combinatorial strategies that could be designed to modulate CGRP signaling for migraine and other diseases.
Keywords: CGRP; GPCR; migraine; neuropeptide; trigeminal nerve.
Conflict of interest statement
D.L.H. has received research support from Living Cell Technologies and AbbVie and has acted as an advisor, speaker, or consultant for Lilly, Teva, Merck, and Amgen. A.F.R. has received research support from Lundbeck and has acted as an advisor or consultant for Lilly, AbbVie, Amgen, Novartis, Lundbeck, and Schedule One Therapeutics.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

