Comprehensive evaluation of human-derived anti-poly-GA antibodies in cellular and animal models of C9orf72 disease
- PMID: 36454749
- PMCID: PMC9894253
- DOI: 10.1073/pnas.2123487119
Comprehensive evaluation of human-derived anti-poly-GA antibodies in cellular and animal models of C9orf72 disease
Abstract
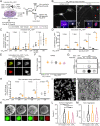
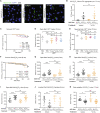
Hexanucleotide G4C2 repeat expansions in the C9orf72 gene are the most common genetic cause of amyotrophic lateral sclerosis and frontotemporal dementia. Dipeptide repeat proteins (DPRs) generated by translation of repeat-containing RNAs show toxic effects in vivo as well as in vitro and are key targets for therapeutic intervention. We generated human antibodies that bind DPRs with high affinity and specificity. Anti-GA antibodies engaged extra- and intra-cellular poly-GA and reduced aggregate formation in a poly-GA overexpressing human cell line. However, antibody treatment in human neuronal cultures synthesizing exogenous poly-GA resulted in the formation of large extracellular immune complexes and did not affect accumulation of intracellular poly-GA aggregates. Treatment with antibodies was also shown to directly alter the morphological and biochemical properties of poly-GA and to shift poly-GA/antibody complexes to more rapidly sedimenting ones. These alterations were not observed with poly-GP and have important implications for accurate measurement of poly-GA levels including the need to evaluate all centrifugation fractions and disrupt the interaction between treatment antibodies and poly-GA by denaturation. Targeting poly-GA and poly-GP in two mouse models expressing G4C2 repeats by systemic antibody delivery for up to 16 mo was well-tolerated and led to measurable brain penetration of antibodies. Long-term treatment with anti-GA antibodies produced improvement in an open-field movement test in aged C9orf72450 mice. However, chronic administration of anti-GA antibodies in AAV-(G4C2)149 mice was associated with increased levels of poly-GA detected by immunoassay and did not significantly reduce poly-GA aggregates or alleviate disease progression in this model.
Keywords: C9orf72; amyotrophic lateral sclerosis; dipeptide repeat proteins; frontotemporal dementia; immunotherapy.
Conflict of interest statement
The authors declare a competing interest. K.D.M., N.C., P.B., C.H., R.M.N., F.M., and J.G. are employees of Neurimmune and S.N., Y.G., S.D., I.D.-L., M.W.K., and M.R. are employees of Biogen.
Figures


References
-
- Mori K., et al. , The C9orf72 Ggggcc repeat is translated into aggregating dipeptide-repeat proteins in Ftld/Als. Science 339, 1335–1338 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

