Trajectories for the evolution of bacterial CO2-concentrating mechanisms
- PMID: 36454757
- PMCID: PMC9894237
- DOI: 10.1073/pnas.2210539119
Trajectories for the evolution of bacterial CO2-concentrating mechanisms
Abstract
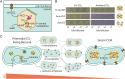
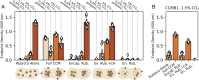
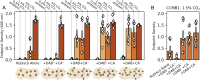
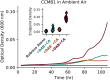
Cyanobacteria rely on CO2-concentrating mechanisms (CCMs) to grow in today's atmosphere (0.04% CO2). These complex physiological adaptations require ≈15 genes to produce two types of protein complexes: inorganic carbon (Ci) transporters and 100+ nm carboxysome compartments that encapsulate rubisco with a carbonic anhydrase (CA) enzyme. Mutations disrupting any of these genes prohibit growth in ambient air. If any plausible ancestral form-i.e., lacking a single gene-cannot grow, how did the CCM evolve? Here, we test the hypothesis that evolution of the bacterial CCM was "catalyzed" by historically high CO2 levels that decreased over geologic time. Using an E. coli reconstitution of a bacterial CCM, we constructed strains lacking one or more CCM components and evaluated their growth across CO2 concentrations. We expected these experiments to demonstrate the importance of the carboxysome. Instead, we found that partial CCMs expressing CA or Ci uptake genes grew better than controls in intermediate CO2 levels (≈1%) and observed similar phenotypes in two autotrophic bacteria, Halothiobacillus neapolitanus and Cupriavidus necator. To understand how CA and Ci uptake improve growth, we model autotrophy as colimited by CO2 and HCO3-, as both are required to produce biomass. Our experiments and model delineated a viable trajectory for CCM evolution where decreasing atmospheric CO2 induces an HCO3- deficiency that is alleviated by acquisition of CA or Ci uptake, thereby enabling the emergence of a modern CCM. This work underscores the importance of considering physiology and environmental context when studying the evolution of biological complexity.
Keywords: Earth history; carbon fixation; evolution; photosynthesis; synthetic biology.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Bathellier C., Tcherkez G., Lorimer G. H., Farquhar G. D., Rubisco is not really so bad. Plant Cell Environ. 41, 705–716 (2018). - PubMed
-
- Iñiguez C., et al. , Evolutionary trends in RuBisCO kinetics and their co-evolution with CO2 concentrating mechanisms. Plant J. 101, 897–918 (2020). - PubMed
-
- Bowes G., Ogren W. L., Hageman R. H., Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 45, 716–722 (1971). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

