The E46K mutation modulates α-synuclein prion replication in transgenic mice
- PMID: 36454879
- PMCID: PMC9714912
- DOI: 10.1371/journal.ppat.1010956
The E46K mutation modulates α-synuclein prion replication in transgenic mice
Abstract
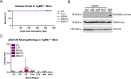
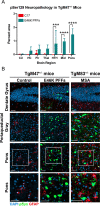
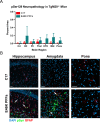
In multiple system atrophy (MSA), the α-synuclein protein misfolds into a self-templating prion conformation that spreads throughout the brain, leading to progressive neurodegeneration. While the E46K mutation in α-synuclein causes familial Parkinson's disease (PD), we previously discovered that this mutation blocks in vitro propagation of MSA prions. Recent studies by others indicate that α-synuclein adopts a misfolded conformation in MSA in which a Greek key motif is stabilized by an intramolecular salt bridge between residues E46 and K80. Hypothesizing that the E46K mutation impedes salt bridge formation and, therefore, exerts a selective pressure that can modulate α-synuclein strain propagation, we asked whether three distinct α-synuclein prion strains could propagate in TgM47+/- mice, which express human α-synuclein with the E46K mutation. Following intracranial injection of these strains, TgM47+/- mice were resistant to MSA prion transmission, whereas recombinant E46K preformed fibrils (PFFs) transmitted neurological disease to mice and induced the formation of phosphorylated α-synuclein neuropathology. In contrast, heterotypic seeding following wild-type (WT) PFF-inoculation resulted in preclinical α-synuclein prion propagation. Moreover, when we inoculated TgM20+/- mice, which express WT human α-synuclein, with E46K PFFs, we observed delayed transmission kinetics with an incomplete attack rate. These findings suggest that the E46K mutation constrains the number of α-synuclein prion conformations that can propagate in TgM47+/- mice, expanding our understanding of the selective pressures that impact α-synuclein prion replication.
Copyright: © 2022 Holec et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Spillantini MG, Schmidt ML, Lee VM-Y, Trojanowski JQ, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–40. - PubMed
-
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

