Systematic review and meta-analysis of the efficacy and safety of oseltamivir (Tamiflu) in the treatment of Coronavirus Disease 2019 (COVID-19)
- PMID: 36454880
- PMCID: PMC9714710
- DOI: 10.1371/journal.pone.0277206
Systematic review and meta-analysis of the efficacy and safety of oseltamivir (Tamiflu) in the treatment of Coronavirus Disease 2019 (COVID-19)
Abstract
Efforts are ongoing by researchers globally to develop new drugs or repurpose existing ones for treating COVID-19. Thus, this led to the use of oseltamivir, an antiviral drug used for treating influenza A and B viruses, as a trial drug for COVID-19. However, available evidence from clinical studies has shown conflicting results on the effectiveness of oseltamivir in COVID-19 treatment. Therefore, this systematic review and meta-analysis was performed to assess the clinical safety and efficacy of oseltamivir for treating COVID-19. The study was conducted according to the PRISMA guidelines, and the priori protocol was registered in PROSPERO (CRD42021270821). Five databases were searched, the identified records were screened, and followed by the extraction of relevant data. Eight observational studies from four Asian countries were included. A random-effects model was used to pool odds ratios (ORs), mean differences (MD), and their 95% confidence intervals (CI) for the study analysis. Survival was not significantly different between all categories of oseltamivir and the comparison groups analysed. The duration of hospitalisation was significantly shorter in the oseltamivir group following sensitivity analysis (MD -5.95, 95% CI -9.91--1.99 p = 0.003, heterogeneity I2 0%, p = 0.37). The virological, laboratory and radiological response rates were all not in favour of oseltamivir. However, the electrocardiographic safety parameters were found to be better in the oseltamivir group. However, more studies are needed to establish robust evidence on the effectiveness or otherwise of oseltamivir usage for treating COVID-19.
Copyright: © 2022 Aliyu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


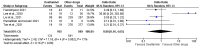





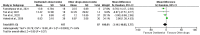
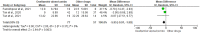

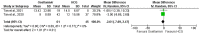

References
-
- WHO, W.H.O., Novel Coronavirus (2019-nCoV): situation report, 22. 2020.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

