Patterns of antiemetic medication use during pregnancy: A multi-country retrospective cohort study
- PMID: 36454900
- PMCID: PMC9714905
- DOI: 10.1371/journal.pone.0277623
Patterns of antiemetic medication use during pregnancy: A multi-country retrospective cohort study
Abstract
Objective: To compare patterns in use of different antiemetics during pregnancy in Canada, the United Kingdom, and the United States, between 2002 and 2014.
Methods: We constructed population-based cohorts of pregnant women using administrative healthcare data from five Canadian provinces (Alberta, British Columbia, Manitoba, Ontario, and Saskatchewan), the Clinical Practice Research Datalink from the United Kingdom, and the IBM MarketScan Research Databases from the United States. We included pregnancies ending in live births, stillbirth, spontaneous abortion, or induced abortion. We determined maternal use of antiemetics from pharmacy claims in Canada and the United States and from prescriptions in the United Kingdom.
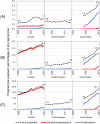
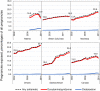
Results: The most common outcome of 3 848 734 included pregnancies (started 2002-2014) was live birth (66.7% of all pregnancies) followed by spontaneous abortion (20.2%). Use of antiemetics during pregnancy increased over time in all three countries. Canada had the highest prevalence of use of prescription antiemetics during pregnancy (17.7% of pregnancies overall, 13.2% of pregnancies in 2002, and 18.9% in 2014), followed by the United States (14.0% overall, 8.9% in 2007, and 18.1% in 2014), and the United Kingdom (5.0% overall, 4.2% in 2002, and 6.5% in 2014). Besides use of antiemetic drugs being considerably lower in the United Kingdom, the increase in its use over time was more modest. The most commonly used antiemetic was combination doxylamine/pyridoxine in Canada (95.2% of pregnancies treated with antiemetics), ondansetron in the United States (72.2%), and prochlorperazine in the United Kingdom (63.5%).
Conclusions: In this large cohort study, we observed an overall increase in antiemetic use during pregnancy, and patterns of use varied across jurisdictions. Continued monitoring of antiemetic use and further research are warranted to better understand the reasons for differences in use of these medications and to assess their benefit-risk profile in this population.
Copyright: © 2022 Fisher et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
: SS reported receiving personal fees from Atara, AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Novartis, Pfizer, and Seqirus outside the submitted work. KBF is supported by a salary support award from the Fonds de recherche du Québec – santé (FRQS; Quebec Foundation for Research – Health) and a William Dawson Scholar award from McGill University. RWP holds the Albert Boehringer I Chair in Pharmacoepidemiology at McGill University. RWP reported receiving personal fees from Amgen, Biogen, Merck, and Pfizer outside the submitted work. MP reported grants from Canadian Institutes of Health Research during the conduct of the study. No other disclosures were reported. No endorsement by the funders, data providers, data stewards, CIHI, or Health Canada is intended or should be inferred. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

