Plant regeneration from leaf mesophyll derived protoplasts of cassava (Manihot esculenta Crantz)
- PMID: 36454974
- PMCID: PMC9714804
- DOI: 10.1371/journal.pone.0278717
Plant regeneration from leaf mesophyll derived protoplasts of cassava (Manihot esculenta Crantz)
Abstract
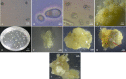
A high yield of isolated protoplast and reliable regeneration system are prerequisite for successful somatic hybridization and genome editing research. However, reproducible plant regeneration from protoplasts remains a bottleneck for many crops, including cassava. We evaluated several factors that influence isolation of viable protoplasts form leaf mesophyll, induction of embryogenic calli, and regeneration of plants in three cassava cultivars; Muchericheri, TMS60444 and Karibuni. A relatively higher protoplast yield was obtained with enzyme mixture containing 5 g/L Macerozyme and 10 g/L cellulase. Muchericheri recorded relatively higher protoplast yield of 20.50±0.50×106 whereas TMS60444 (10.25±0.25×106) had the least protoplast yield in 10 g/L cellulase and 4 g/L cellulase. Freshly isolated protoplast cells were plated on callus induction medium (CIM) solid medium containing MS basal salt, 60 g/L D-glucose, 30 g/L sucrose, B5 vitamins, 100 mg/L myo-inositol, 0.5 mg/L copper sulphate, 100 mg/L casein hydrolysate, 4.55 g/L mannitol, 0.1 g/L MES, 10 mg/L picloram and 3 g/L gelrite to induce protoplast growth and development. The three cultivars reached colony formation but no further development was observed in this culture method. Protoplast growth and development was further evaluated in suspension culture using varying cell densities (1, 2 and 3× 105 p/mL). Development with highest number of minicalli was observed in cell density of 3× 105 p/mL. Minicalli obtained were cultured on CIM supplemented with 10mg/L picloram. Callus induction was observed in all cell densities with the cultivars. Highest somatic embryogenesis was observed in 2× 105 p/ml while no somatic embryogenesis was observed in cell density of 1×105 p/mL. Somatic embryos were matured in EMM medium supplemented with 1 mg/L BAP, 0.02 mg/L NAA and 1.5 mg/L GA3 then germinated in hormone free medium for plant regeneration. This protocol which used simple mixture of commercial enzymes is highly reproducible and can be applied in biotechnology research on cassava.
Copyright: © 2022 Mukami et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures



Similar articles
-
Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz).BMC Biotechnol. 2017 Mar 14;17(1):29. doi: 10.1186/s12896-017-0349-2. BMC Biotechnol. 2017. PMID: 28292294 Free PMC article.
-
Plant regeneration from protoplasts isolated from embryogenic calli of the forage legume Astragalus melilotoides Pall.Plant Cell Rep. 2004 May;22(10):741-6. doi: 10.1007/s00299-004-0760-8. Epub 2004 Feb 5. Plant Cell Rep. 2004. PMID: 14762684
-
Plant regeneration from protoplasts isolated from embryogenic suspension cultured cells of Cinnamomum camphora L.Plant Cell Rep. 2005 Oct;24(8):462-7. doi: 10.1007/s00299-005-0969-1. Epub 2005 Jun 7. Plant Cell Rep. 2005. PMID: 15940527
-
Efficient regeneration of protoplasts from Solanum lycopersicum cultivar Micro-Tom.Biol Methods Protoc. 2024 Feb 6;9(1):bpae008. doi: 10.1093/biomethods/bpae008. eCollection 2024. Biol Methods Protoc. 2024. PMID: 38414647 Free PMC article. Review.
-
Protoplast Technology and Somatic Hybridisation in the Family Apiaceae.Plants (Basel). 2023 Feb 27;12(5):1060. doi: 10.3390/plants12051060. Plants (Basel). 2023. PMID: 36903923 Free PMC article. Review.
Cited by
-
Advancements and strategies of genetic improvement in cassava (Manihot esculenta Crantz): from conventional to genomic approaches.Hortic Res. 2024 Dec 2;12(3):uhae341. doi: 10.1093/hr/uhae341. eCollection 2025 Mar. Hortic Res. 2024. PMID: 40061801 Free PMC article.
-
Establishing a reliable protoplast system for grapevine: isolation, transformation, and callus induction.Protoplasma. 2025 Apr 25. doi: 10.1007/s00709-025-02069-7. Online ahead of print. Protoplasma. 2025. PMID: 40278881
-
Advances in Quercus ilex L. breeding: the CRISPR/Cas9 technology via ribonucleoproteins.Front Plant Sci. 2024 Feb 19;15:1323390. doi: 10.3389/fpls.2024.1323390. eCollection 2024. Front Plant Sci. 2024. PMID: 38439988 Free PMC article.
-
Modern Technologies Provide New Opportunities for Somatic Hybridization in the Breeding of Woody Plants.Plants (Basel). 2024 Sep 10;13(18):2539. doi: 10.3390/plants13182539. Plants (Basel). 2024. PMID: 39339514 Free PMC article. Review.
-
Optimization of preparation and transformation of protoplasts from Populus simonii × P. nigra leaves and subcellular localization of the major latex protein 328 (MLP328).Plant Methods. 2024 Jan 4;20(1):3. doi: 10.1186/s13007-023-01128-5. Plant Methods. 2024. PMID: 38178205 Free PMC article.
References
-
- FAO, 2013. FAOSTAT Database. Food and Agriculture Organization. Rome, Italy. Available online at URL: www.fao.org.
-
- Karuri E. E., Mbugua S. K., Karugia J., Wanda K., & Jagwe J. (2001). Marketing Opportunities for cassava-based products: An Assessment of the Industrial Potential in Kenya. Cassava Industrial Demand Analysis in Kenya, 1–59.
-
- United Nations (UN) (2010). Keeping the promise: United to achieve the Millennium Development Goals (A/RES/65/1). Available at: https://www.unwomen.org/en/docs/2010/10/un-general-assembly-resolution-65-1 (accessed 21 August 2022).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

