The adverse effects of trastuzumab-containing regimes as a therapy in breast cancer: A piggy-back systematic review and meta-analysis
- PMID: 36454979
- PMCID: PMC9714930
- DOI: 10.1371/journal.pone.0275321
The adverse effects of trastuzumab-containing regimes as a therapy in breast cancer: A piggy-back systematic review and meta-analysis
Abstract
Background: Trastuzumab is a valuable therapy option for women with ERBB2(HER2)+ breast cancer tumours, often used in combination with chemotherapy and alongside other therapies. It is known to have adverse effects, but these have proved difficult to separate from the effects of other concurrent therapies patients are usually taking. This study aims to assess the adverse effects specifically attributable to trastuzumab, and whether they vary by patient subgroup or concurrent therapies.
Methods: As registered on PROSPERO (CRD42019146541), we used previous systematic reviews as well as the clinicaltrials.gov registry to identify randomised controlled trials in breast cancer which compared treatment regimes with and without trastuzumab. Neoadjuvant, adjuvant and metastatic settings were examined. Data was extracted from those which had, as of July 2022, reported adverse events. Risk of bias was assessed using ROB2. Primary outcomes were adverse events of any type or severity (excluding death). A standard random-effects meta-analysis was performed for each outcome independently. In order to ascertain whether adverse effects differed by individual factors such as age or tumour characteristics, or by use of trastuzumab concurrently with hormone therapy, we examined individual-level patient data for one large trial, HERA.
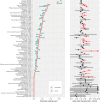
Results: 79 relevant trials were found, of which 20 contained comparable arms of trastuzumab-containing therapy and corresponding matched therapy without trastuzumab. This allowed a comparison of 8669 patients receiving trastuzumab versus 9556 receiving no trastuzumab, which gave a list of 25 statistically and clinically significant adverse effects related to trastuzumab alone: unspecified pain, asthenia, nasopharyngitis, skin disorders (mainly rash), dyspepsia, paraesthesia, infections (often respiratory), increased lacrimation, diarrhoea, myalgia, oedema (limb/peripheral), fever, nose bleeds, cardiac events, insomnia, cough, back pain, dyspnoea, chills, dizziness or vertigo, hypertension, congestive heart failure, increased levels of aspartate aminotransferase, gastrointestinal issues and dehydration. Analysis of individual patient-level data from 5102 patients suggested that nausea is slightly more likely for women taking trastuzumab who are ER+ /also taking hormone therapy than for those who are ER-/not taking hormone therapy; no other potential treatment-subgroup interactions were detected. We found no evidence for significantly increased rates of neutropenia, anaemia or lymphopenia in patients on trastuzumab-containing regimes compared to those on comparable regimes without trastuzumab.
Conclusions: This meta-analysis should allow clinicians and patients to better identify and quantify the potential adverse effects of adding trastuzumab to their treatment regime for breast cancer, and hence inform their decision-making. However, limitations include serious risk of bias due to heterogeneity in reporting of the outcomes and the open-label nature of the trials.
Copyright: © 2022 Jackson et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Baselga BJ, Tripathy D, Mendelsohn J, et al. (1996) Phase II Study of Weekly Intravenous Recombinant Metastatic Breast Cancer. J Clin Oncol 14:737–744 - PubMed
-
- Food and drugs administration (2010) HERCEPTIN® (Trastuzumab) Final labelling evidence.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

