Analysis of residual disease in periocular basal cell carcinoma following hedgehog pathway inhibition: Follow up to the VISORB trial
- PMID: 36455049
- PMCID: PMC9714843
- DOI: 10.1371/journal.pone.0265212
Analysis of residual disease in periocular basal cell carcinoma following hedgehog pathway inhibition: Follow up to the VISORB trial
Abstract
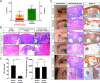
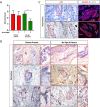
Basal cell carcinoma (BCC) is a common skin cancer caused by deregulated hedgehog signaling. BCC is often curable surgically; however, for orbital and periocular BCCs (opBCC), surgical excision may put visual function at risk. Our recent clinical trial highlighted the utility of vismodegib for preserving visual organs in opBCC patients: 67% of patients displayed a complete response histologically. However, further analysis of excision samples uncovered keratin positive, hedgehog active (Gli1 positive), proliferative micro-tumors. Sequencing of pre-treatment tumors revealed resistance conferring mutations present at low frequency. In addition, one patient with a low-frequency SMO W535L mutation recurred two years post study despite no clinical evidence of residual disease. Sequencing of this recurrent tumor revealed an enrichment for the SMO W535L mutation, revealing that vismodegib treatment enriched for resistant cells undetectable by traditional histology. In the age of targeted therapies, linking molecular genetic analysis to prospective clinical trials may be necessary to provide mechanistic understanding of clinical outcomes. Trial Registration: ClinicalTrials.gov Identifier: NCT02436408.
Copyright: © 2022 Unsworth et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
AK was a consultant to Genentech, Inc., in 2018 and 2019 for the purpose of discussions with the Food and Drug Administration. AK has also had a consulting relationship with Stryker Corporation and BioTissue, Inc. over the past 12 months. SPU, CFT, CJH, ESE, CAA, MPC, and SCB have no relevant conflict of interest disclosures. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Johnson R.L., et al.., Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science, 1996. 272(5268): p. 1668–71. - PubMed
-
- Xie J., et al.., Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature, 1998. 391(6662): p. 90–2. - PubMed
-
- Axelson M., et al.., U.S. Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res, 2013. 19(9): p. 2289–93. - PubMed
-
- Von Hoff D.D., et al.., Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med, 2009. 361(12): p. 1164–72. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

