Safety and Efficacy of MEDI0457 plus Durvalumab in Patients with Human Papillomavirus-Associated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
- PMID: 36455147
- PMCID: PMC9890138
- DOI: 10.1158/1078-0432.CCR-22-1987
Safety and Efficacy of MEDI0457 plus Durvalumab in Patients with Human Papillomavirus-Associated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma
Abstract
Purpose: Tumoral programmed cell death ligand-1 (PD-L1) expression is common in human papillomavirus (HPV)-associated head and neck squamous cell carcinoma (HNSCC). We assessed whether a DNA vaccine targeting HPV-16/18 E6/E7 with IL12 adjuvant (MEDI0457) combined with the PD-L1 inhibitor durvalumab could enhance HPV-specific T-cell response and improve outcomes in recurrent/metastatic HPV-16/18-associated HNSCC.
Patients and methods: In this phase Ib/IIa study, immunotherapy-naïve patients with ≥1 previous platinum-containing regimen (neoadjuvant/adjuvant therapy or for recurrent/metastatic disease) received MEDI0457 7 mg intramuscularly with electroporation on weeks 1, 3, 7, and 12, then every 8 weeks, plus durvalumab 1,500 mg intravenously on weeks 4, 8, and 12, then every 4 weeks, until confirmed progression and/or unacceptable toxicity. Coprimary objectives were safety and objective response rate (ORR; H0: ORR ≤ 15%); secondary objectives included 16-week disease control rate (DCR-16), overall survival (OS), and progression-free survival (PFS).
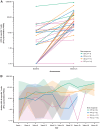
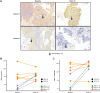
Results: Of 35 treated patients, 29 were response evaluable (confirmed HPV-associated disease; received both agents). ORR was 27.6% [95% confidence interval (CI), 12.7-47.2; four complete responses, four partial responses]; responses were independent of PD-L1 tumor-cell expression (≥25% vs. <25%). DCR-16 was 44.8% (95% CI, 26.5-64.3). Median PFS was 3.5 months (95% CI, 1.9-9.0); median OS was 29.2 months (15.2-not calculable). Twenty-eight (80.0%) patients had treatment-related adverse events [grade 3: 5 (14.3%); no grade 4/5], resulting in discontinuation in 2 (5.7%) patients. HPV-16/18-specific T cells increased on treatment; 4 of 8 evaluable patients had a >2-fold increase in tumor-infiltrating CD8+ T cells.
Conclusions: MEDI0457 plus durvalumab was well tolerated. While the primary efficacy endpoint was not reached, clinical benefit was encouraging.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

Comment in
-
MEDI0457 Plus Durvalumab in HPV-associated HNSCC-Letter.Clin Cancer Res. 2023 Jul 14;29(14):2735. doi: 10.1158/1078-0432.CCR-23-0895. Clin Cancer Res. 2023. PMID: 37449359 No abstract available.
References
-
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–75. - PubMed
-
- Future IIS Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356:1915–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

