Pembrolizumab Versus Placebo as Second-Line Therapy in Patients From Asia With Advanced Hepatocellular Carcinoma: A Randomized, Double-Blind, Phase III Trial
- PMID: 36455168
- PMCID: PMC9995104
- DOI: 10.1200/JCO.22.00620
Pembrolizumab Versus Placebo as Second-Line Therapy in Patients From Asia With Advanced Hepatocellular Carcinoma: A Randomized, Double-Blind, Phase III Trial
Abstract
Purpose: We evaluated the efficacy and safety of pembrolizumab in patients from Asia with previously treated advanced hepatocellular carcinoma (HCC).
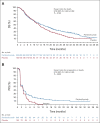
Methods: In a double-blind, phase III trial, 453 patients with advanced HCC and progression during or after treatment with or intolerance to sorafenib or oxaliplatin-based chemotherapy were randomly assigned in a 2:1 ratio to receive pembrolizumab (200 mg) or placebo once every 3 weeks for ≤ 35 cycles plus best supportive care. The primary end point was overall survival (one-sided significance threshold, P = .0193 [final analysis]). Secondary end points included progression-free survival (PFS) and objective response rate (ORR; one-sided significance threshold, P = .0134 and .0091, respectively [second interim analysis]; RECIST version 1.1, by blinded independent central review).
Results: Median overall survival was longer in the pembrolizumab group than in the placebo group (14.6 v 13.0 months; hazard ratio for death, 0.79; 95% CI, 0.63 to 0.99; P = .0180). Median PFS was also longer in the pembrolizumab group than in the placebo group (2.6 v 2.3 months; hazard ratio for progression or death, 0.74; 95% CI, 0.60 to 0.92; P = .0032). ORR was greater in the pembrolizumab group (12.7% [95% CI, 9.1 to 17.0]) than in the placebo group (1.3% [95% CI, 0.2 to 4.6]; P < .0001). Treatment-related adverse events occurred in 66.9% of patients (grade 3, 12.0%; grade 4, 1.3%; grade 5, 1.0%) in the pembrolizumab group and 49.7% of patients (grade 3, 5.9%; grade 4, 0%; grade 5, 0%) in the placebo group.
Conclusion: In patients from Asia with previously treated advanced HCC, pembrolizumab significantly prolonged overall survival and PFS, and ORR was greater versus placebo.
Trial registration: ClinicalTrials.gov NCT03062358.
Figures
Comment in
-
Determining the Efficacy of Pembrolizumab in Patients With Previously Treated Advanced Hepatocellular Carcinoma.J Clin Oncol. 2023 Jun 1;41(16):3074-3075. doi: 10.1200/JCO.23.00072. Epub 2023 Apr 4. J Clin Oncol. 2023. PMID: 37015029 Free PMC article. No abstract available.
References
-
- World Health Organization : GLOBOCAN 2020 – Liver Cancer Factsheet. Geneva, World Health Organization, 2020
-
- Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 - PubMed
-
- Finn RS, Qin S, Ikeda M, et al. : Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 382:1894-1905, 2020 - PubMed
-
- Ren Z, Xu J, Bai Y, et al. : Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol 22:977-990, 2021 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical