DDX41-associated susceptibility to myeloid neoplasms
- PMID: 36455200
- PMCID: PMC10356560
- DOI: 10.1182/blood.2022017715
DDX41-associated susceptibility to myeloid neoplasms
Abstract
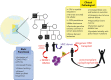
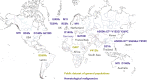
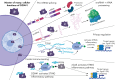
Deleterious germ line DDX41 variants confer risk for myeloid neoplasms (MNs) and less frequently for lymphoid malignancies, with autosomal dominant inheritance and an estimated prevalence of 3% among MNs. Germ line DDX41 variants include truncating alleles that comprise about two-thirds of all alleles, missense variants located preferentially within the DEAD-box domain, and deletion variants. The identification of a truncating allele on tumor-based molecular profiling should prompt germ line genetic testing because >95% of such alleles are germ line. Somatic mutation of the wild-type DDX41 allele occurs in about half of MNs with germ line DDX41 alleles, typically in exons encoding the helicase domain and most frequently as R525H. Several aspects of deleterious germ line DDX41 alleles are noteworthy: (1) certain variants are common in particular populations, (2) MNs develop at older ages typical of de novo disease, challenging the paradigm that inherited cancer risk always causes disease in young people, (3) despite equal frequencies of these variants in men and women, men progress to MNs more frequently, suggesting a gender-specific effect on myeloid leukemogenesis, and (4) individuals with deleterious germ line DDX41 variants develop acute severe graft-versus-host disease after allogeneic hematopoietic cell transplantation with wild-type donors more than others unless they receive posttransplant cyclophosphamide, suggesting a proinflammatory milieu that stimulates donor-derived T cells. Biochemical studies and animal models have identified DDX41's ability to interact with double-stranded DNA and RNA:DNA hybrids with roles in messenger RNA splicing, ribosomal RNAs or small nucleolar RNAs processing, and modulation of innate immunity, disruption of which could promote inflammation and drive tumorigenesis.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: L.A.G. receives royalties from UptoDate, Inc for a coauthored article on germ line predisposition to hematopoietic malignancies. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Introduction to a review series on germ line predisposition to hematologic malignancies: time to consider germ line testing.Blood. 2023 Mar 30;141(13):1509-1512. doi: 10.1182/blood.2023019846. Blood. 2023. PMID: 36787501 No abstract available.
References
-
- Makishima H, Saiki R, Nannya Y, et al. Germ line DDX41 mutations define a unique subtype of myeloid neoplasm. Blood. 2023;141(5):534–549. - PubMed
-
- Quesada AE, Routbort MJ, DiNardo CD, et al. DDX41 mutations in myeloid neoplasms are associated with male gender, TP53 mutations and high-risk disease. Am J Hematol. 2019;94(7):757–766. - PubMed
-
- Goyal T, Tu ZJ, Wang Z, Cook JR. Clinical and pathologic spectrum of DDX41-mutated hematolymphoid neoplasms. Am J Clin Pathol. 2021;156(5):829–838. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

