Pooled safety analysis from phase III studies of trifluridine/tipiracil in patients with metastatic gastric or gastroesophageal junction cancer and metastatic colorectal cancer
- PMID: 36455504
- PMCID: PMC9808443
- DOI: 10.1016/j.esmoop.2022.100633
Pooled safety analysis from phase III studies of trifluridine/tipiracil in patients with metastatic gastric or gastroesophageal junction cancer and metastatic colorectal cancer
Abstract
Background: Trifluridine/tipiracil (FTD/TPI) showed clinical benefit, including improved survival and manageable safety in previously treated patients with metastatic colorectal (mCRC) or gastric/gastroesophageal junction (mGC/GEJC) cancer in the phase III RECOURSE and TAGS trials, respectively. A pooled analysis was conducted to further characterize FTD/TPI safety, including management of haematologic toxicities and use in patients with renal or hepatic impairment.
Patients and methods: Adults with ≥2 prior regimens for advanced mGC/GEJC or mCRC were randomized (2 : 1) to FTD/TPI [35 mg/m2 twice daily days 1-5 and 8-12 (28-day cycle); same dosage in both trials] or placebo plus best supportive care. Adverse events (AEs) were summarized in the safety population (patients who received ≥1 dose) and analysed by renal/hepatic function.
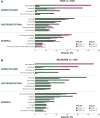
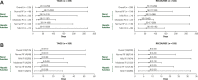
Results: TAGS and RECOURSE included 335 and 533 FTD/TPI-treated and 168 and 265 placebo-treated patients, respectively. Overall safety of FTD/TPI was similar in TAGS and RECOURSE. Haematologic (neutropenia, anaemia) and gastrointestinal (nausea, diarrhoea) AEs were most commonly observed. Laboratory-assessed grade 3-4 neutropenia occurred in 37% (TAGS)/38% (RECOURSE) of FTD/TPI-treated patients (median onset: 29 days/55 days), and 96% (TAGS)/97% (RECOURSE) of cases resolved regardless of renal/hepatic function. Supportive medications for neutropenia were received by 17% (TAGS) and 9% (RECOURSE); febrile neutropenia was reported in 2% and 4%, respectively. Overall grade ≥3 AEs were more frequent in patients with moderate renal impairment [81% (TAGS); 85% (RECOURSE)] versus normal renal function (74%; 67%); anaemia and neutropenia were more common in patients with renal impairment. FTD/TPI safety (including haematologic AEs) was consistent across patients with normal and mildly impaired hepatic function.
Conclusions: These results support FTD/TPI as a well-tolerated treatment in patients with mGC/GEJC or mCRC, with a consistent safety profile. Safety was largely similar in patients with normal or mildly impaired renal/hepatic function; however, patients with renal impairment should be monitored for haematologic toxicities.
Keywords: metastatic colorectal cancer; metastatic gastric cancer; neutropenia; renal impairment; safety; trifluridine/tipiracil.
Copyright © 2022. Published by Elsevier Ltd.
Figures

References
-
- Emura T., Suzuki N., Yamaguchi M., Ohshimo H., Fukushima M. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol. 2004;25(3):571–578. - PubMed
-
- Fukushima M., Suzuki N., Emura T., et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2’-deoxyribonucleosides. Biochem Pharmacol. 2000;59(10):1227–1236. - PubMed
-
- Emura T., Murakami Y., Nakagawa F., Fukushima M., Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med. 2004;13(4):545–549. - PubMed
-
- LONSURF® (trifluridine and tipiracil) tablets [prescribing information] Taiho Oncology Inc; Princeton, NJ: 2019.
-
- Mayer R.J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909–1919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

