Identification of natural products and FDA-approved drugs for targeting cancer stem cell (CSC) propagation
- PMID: 36455875
- PMCID: PMC9792210
- DOI: 10.18632/aging.204412
Identification of natural products and FDA-approved drugs for targeting cancer stem cell (CSC) propagation
Abstract
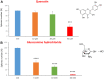
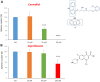
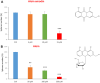
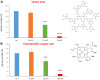
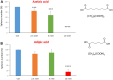
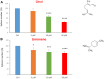
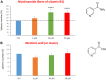
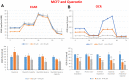
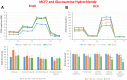
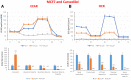
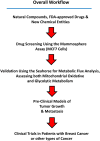
Here, we report the identification of key compounds that effectively inhibit the anchorage-independent growth and propagation of cancer stem cells (CSCs), as determined via screening using MCF7 cells, a human breast adenocarcinoma cell line. More specifically, we employed the mammosphere assay as an experimental format, which involves the generation of 3D spheroid cultures, using low-attachment plates. These positive hit compounds can be divided into 5 categories: 1) dietary supplements (quercetin and glucosamine); 2) FDA-approved drugs (carvedilol and ciprofloxacin); 3) natural products (aloe emodin, aloin, tannic acid, chlorophyllin copper salt, azelaic acid and adipic acid); 4) flavours (citral and limonene); and 5) vitamins (nicotinamide and nicotinic acid). In addition, for the compounds quercetin, glucosamine and carvedilol, we further assessed their metabolic action, using the Seahorse to conduct metabolic flux analysis. Our results indicate that these treatments can affect glycolytic flux and suppress oxidative mitochondrial metabolism (OXPHOS). Therefore, quercetin, glucosamine and carvedilol can reprogram the metabolic phenotype of breast cancer cells. Despite having diverse chemical structures, these compounds all interfere with mitochondrial metabolism. As these compounds halt CSCs propagation, ultimately, they may have therapeutic potential.
Keywords: FDA approved drugs; cancer stem cells; drug screening; mammospheres; natural compounds.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

