Elexacaftor/tezacaftor/ivacaftor corrects monocyte microbicidal deficiency in cystic fibrosis
- PMID: 36455959
- PMCID: PMC10066567
- DOI: 10.1183/13993003.00725-2022
Elexacaftor/tezacaftor/ivacaftor corrects monocyte microbicidal deficiency in cystic fibrosis
Abstract
Background: Cystic fibrosis (CF), which is caused by mutations in the CF transmembrane conductance regulator (CFTR), is characterised by chronic bacterial lung infection and inflammation. In CF, monocytes and monocyte-derived macrophages have been shown to display defective phagocytosis and antimicrobial activity against relevant lung pathogens, including Pseudomonas aeruginosa. Thus, we addressed the effect of CFTR triple modulator therapy (elexacaftor/tezacaftor/ivacaftor (ETI)) on the activity of CF monocytes against P. aeruginosa.
Methods: Monocytes from people with CF (PWCF) before and after 1 and 6 months of ETI therapy were isolated from blood and infected with P. aeruginosa to assess phagocytic activity and intracellular bacterial killing. The oxidative burst and interleukin-6 secretion were also determined. Monocytes from healthy controls were also included.
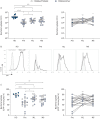
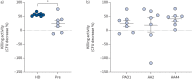
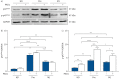
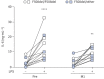
Results: Longitudinal analysis of the clinical parameters confirmed an improvement of lung function and lung microbiology by ETI. Both the phagocytic and microbicidal deficiencies of CF monocytes also improved significantly, although not completely. Furthermore, we measured an exuberant oxidative burst in CF monocytes before therapy, which was reduced considerably by ETI. This led to an improvement of reactive oxygen species-dependent bactericidal activity. Inflammatory response to bacterial stimuli was also lowered compared with pre-therapy.
Conclusions: PWCF on ETI therapy, in a real-life setting, in addition to clinical recovery, showed significant improvement in monocyte activity against P. aeruginosa, which may have contributed to the overall effect of ETI on pulmonary disease. This also suggests that CF monocyte dysfunctions may be specifically targeted to ameliorate lung function in CF.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: All authors have nothing to disclose.
Figures



Comment in
-
The effects of elexafactor/tezafactor/ivacaftor beyond the epithelium: spurring macrophages to fight infections.Eur Respir J. 2023 Apr 1;61(4):2300216. doi: 10.1183/13993003.00216-2023. Print 2023 Apr. Eur Respir J. 2023. PMID: 37003613 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
