Rapamycin and inulin for third-dose vaccine response stimulation (RIVASTIM): Inulin - study protocol for a pilot, multicentre, randomised, double-blinded, controlled trial of dietary inulin to improve SARS-CoV-2 vaccine response in kidney transplant recipients
- PMID: 36456021
- PMCID: PMC9716412
- DOI: 10.1136/bmjopen-2022-062747
Rapamycin and inulin for third-dose vaccine response stimulation (RIVASTIM): Inulin - study protocol for a pilot, multicentre, randomised, double-blinded, controlled trial of dietary inulin to improve SARS-CoV-2 vaccine response in kidney transplant recipients
Abstract
Introduction: Kidney transplant recipients (KTRs) are at an increased risk of hospitalisation and death from COVID-19. Vaccination against SARS-CoV-2 is our primary risk mitigation strategy, yet vaccine effectiveness in KTRs is suboptimal. Strategies to enhance vaccine efficacy are therefore required. Current evidence supports the role of the gut microbiota in shaping the immune response to vaccination. Gut dysbiosis is common in KTRs and is a potential contributor to impaired COVID-19 vaccine responses. We hypothesise that dietary fibre supplementation will attenuate gut dysbiosis and promote vaccine responsiveness in KTRs.
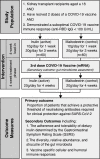
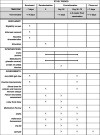
Methods and analysis: Rapamycin and inulin for third-dose vaccine response stimulation-inulin is a multicentre, randomised, prospective, double-blinded, placebo-controlled pilot trial examining the effect of dietary inulin supplementation prior to a third dose of COVID-19 vaccine in KTRs who have failed to develop protective immunity following a 2-dose COVID-19 vaccine schedule. Participants will be randomised 1:1 to inulin (active) or maltodextrin (placebo control), administered as 20 g/day of powdered supplement dissolved in water, for 4 weeks prior to and following vaccination. The primary outcome is the proportion of participants in each trial arm that achieve in vitro neutralisation of live SARS-CoV-2 virus at 4 weeks following a third dose of COVID-19 vaccine. Secondary outcomes include the safety and tolerability of dietary inulin, the diversity and differential abundance of gut microbiota, and vaccine-specific immune cell populations and responses.
Ethics and dissemination: Ethics approval was obtained from the Central Adelaide Local Health Network (CALHN) Human Research Ethics Committee (HREC) (approval number: 2021/HRE00354) and the Sydney Local Health District (SHLD) HREC (approval numbers: X21-0411 and 2021/STE04280). Results of this trial will be published following peer-review and presented at scientific meetings and congresses.
Trial registration number: ACTRN12621001465842.
Keywords: COVID-19; IMMUNOLOGY; NUTRITION & DIETETICS; Renal transplantation; Transplant medicine; VIROLOGY.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Dietary Inulin to Improve SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients: The RIVASTIM-Inulin Randomised Controlled Trial.Vaccines (Basel). 2024 Jun 3;12(6):608. doi: 10.3390/vaccines12060608. Vaccines (Basel). 2024. PMID: 38932337 Free PMC article.
-
Rapamycin and inulin for booster vaccine response stimulation (RIVASTIM)-rapamycin: study protocol for a randomised, controlled trial of immunosuppression modification with rapamycin to improve SARS-CoV-2 vaccine response in kidney transplant recipients.Trials. 2022 Sep 15;23(1):780. doi: 10.1186/s13063-022-06634-w. Trials. 2022. PMID: 36109788 Free PMC article.
-
A randomized, double-blind, placebo-controlled phase III clinical trial to evaluate the efficacy and safety of SARS-CoV-2 vaccine (inactivated, Vero cell): a structured summary of a study protocol for a randomised controlled trial.Trials. 2021 Apr 13;22(1):276. doi: 10.1186/s13063-021-05180-1. Trials. 2021. PMID: 33849629 Free PMC article.
-
A Deferred-Vaccination Design to Assess Durability of COVID-19 Vaccine Effect After the Placebo Group Is Vaccinated.Ann Intern Med. 2021 Aug;174(8):1118-1125. doi: 10.7326/M20-8149. Epub 2021 Apr 13. Ann Intern Med. 2021. PMID: 33844575 Free PMC article.
-
Effectiveness, Immunogenicity and Harms of Additional SARS-CoV-2 Vaccine Doses in Kidney Transplant Recipients: A Systematic Review.Vaccines (Basel). 2023 Apr 18;11(4):863. doi: 10.3390/vaccines11040863. Vaccines (Basel). 2023. PMID: 37112775 Free PMC article. Review.
Cited by
-
Dietary Inulin to Improve SARS-CoV-2 Vaccine Response in Kidney Transplant Recipients: The RIVASTIM-Inulin Randomised Controlled Trial.Vaccines (Basel). 2024 Jun 3;12(6):608. doi: 10.3390/vaccines12060608. Vaccines (Basel). 2024. PMID: 38932337 Free PMC article.
-
Immunomodulatory effects of gut microbiota on vaccine efficacy against respiratory pathogens.Front Immunol. 2025 Jun 3;16:1618921. doi: 10.3389/fimmu.2025.1618921. eCollection 2025. Front Immunol. 2025. PMID: 40529354 Free PMC article. Review.
-
Characterization of gut microbiota and metabolites in renal transplant recipients during COVID-19 and prediction of one-year allograft function.J Transl Med. 2025 Apr 10;23(1):420. doi: 10.1186/s12967-025-06090-5. J Transl Med. 2025. PMID: 40211390 Free PMC article.
-
A comprehensive perspective on the interaction between gut microbiota and COVID-19 vaccines.Gut Microbes. 2023 Jan-Dec;15(1):2233146. doi: 10.1080/19490976.2023.2233146. Gut Microbes. 2023. PMID: 37431857 Free PMC article. Review.
-
Intestinal Microbiota and Vaccinations: A Systematic Review of the Literature.Vaccines (Basel). 2025 Mar 12;13(3):306. doi: 10.3390/vaccines13030306. Vaccines (Basel). 2025. PMID: 40266208 Free PMC article. Review.
References
-
- Geneva: World Health Organization . Who COVID-19 Dashboard, 2020. Available: https://covid19.who.int/ [Accessed 17 Jan 2022].
-
- Registry A. 43Rd report chapter 7: transplantation, 2020. Available: https://www.anzdata.org.au
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
