Efficacy and Safety of N-Acetyl-l-Leucine in Children and Adults With GM2 Gangliosidoses
- PMID: 36456200
- PMCID: PMC9990862
- DOI: 10.1212/WNL.0000000000201660
Efficacy and Safety of N-Acetyl-l-Leucine in Children and Adults With GM2 Gangliosidoses
Abstract
Background and objectives: GM2 gangliosidoses (Tay-Sachs and Sandhoff diseases) are rare, autosomal recessive, neurodegenerative diseases with no available symptomatic or disease-modifying treatments. This clinical trial investigated N-acetyl-l-leucine (NALL), an orally administered, modified amino acid in pediatric (≥6 years) and adult patients with GM2 gangliosidoses.
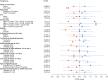
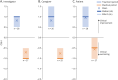
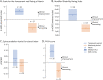
Methods: In this phase IIb, multinational, open-label, rater-blinded study (IB1001-202), male and female patients aged ≥6 years with a genetically confirmed diagnosis of GM2 gangliosidoses received orally administered NALL for a 6-week treatment period (4 g/d in patients ≥13 years, weight-tiered doses for patients 6-12 years), followed by a 6-week posttreatment washout period. For the primary Clinical Impression of Change in Severity analysis, patient performance on a predetermined primary anchor test (the 8-Meter Walk Test or the 9-Hole Peg Test) at baseline, after 6 weeks on NALL, and again after a 6-week washout period was videoed and evaluated centrally by blinded raters. Secondary outcomes included assessments of ataxia, clinical global impression, and quality of life.
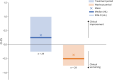
Results: Thirty patients between the age of 6 and 55 years were enrolled. Twenty-nine had an on-treatment assessment and were included in the primary modified intention-to-treat analysis. The study met its CI-CS primary end point (mean difference 0.71, SD = 2.09, 90% CI 0.00, 1.50, p = 0.039), as well as secondary measures of ataxia and global impression. NALL was safe and well tolerated, with no serious adverse reactions.
Discussion: Treatment with NALL was associated with statistically significant and clinically relevant changes in functioning and quality of life in patients with GM2 gangliosidosis. NALL was safe and well tolerated, contributing to an overall favorable risk:benefit profile. NALL is a promising, easily administered (oral) therapeutic option for these rare, debilitating diseases with immense unmet medical needs.
Trial registration information: The trial is registered with ClinicalTrials.gov (NCT03759665; registered on November 30, 2018), EudraCT (2018-004406-25), and DRKS (DRKS00017539). The first patient was enrolled on June 7, 2019.
Classification of evidence: This study provides Class IV evidence that NALL improves outcomes for patients with GM2 gangliosidoses.
© 2022 American Academy of Neurology.
Figures
References
-
- Orphanet. Prevalence and incidence of rare diseases. 2017. Accessed February 15, 2020. www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_al....
-
- Fields T, Patterson M, Bremova-Ertl T, et al. . A master protocol to investigate a novel therapy acetyl-L-leucine for three ultra-rare neurodegenerative diseases: Niemann-Pick type C, the GM2 gangliosidoses, and ataxia telangiectasia. Trials 2021;22(1):84. doi: 10.1186/s13063-020-05009-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
