Electron cryo-microscopy reveals the structure of the archaeal thread filament
- PMID: 36456543
- PMCID: PMC9715654
- DOI: 10.1038/s41467-022-34652-4
Electron cryo-microscopy reveals the structure of the archaeal thread filament
Abstract
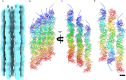
Pili are filamentous surface extensions that play roles in bacterial and archaeal cellular processes such as adhesion, biofilm formation, motility, cell-cell communication, DNA uptake and horizontal gene transfer. The model archaeaon Sulfolobus acidocaldarius assembles three filaments of the type-IV pilus superfamily (archaella, archaeal adhesion pili and UV-inducible pili), as well as a so-far uncharacterised fourth filament, named "thread". Here, we report on the cryo-EM structure of the archaeal thread. The filament is highly glycosylated and consists of subunits of the protein Saci_0406, arranged in a head-to-tail manner. Saci_0406 displays structural similarity, but low sequence homology, to bacterial type-I pilins. Thread subunits are interconnected via donor strand complementation, a feature reminiscent of bacterial chaperone-usher pili. However, despite these similarities in overall architecture, archaeal threads appear to have evolved independently and are likely assembled by a distinct mechanism.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests
Figures


Similar articles
-
Towards a molecular picture of the archaeal cell surface.Nat Commun. 2024 Nov 29;15(1):10401. doi: 10.1038/s41467-024-53986-9. Nat Commun. 2024. PMID: 39614099 Free PMC article.
-
Archaeal type IV pili and their involvement in biofilm formation.Front Microbiol. 2015 Mar 24;6:190. doi: 10.3389/fmicb.2015.00190. eCollection 2015. Front Microbiol. 2015. PMID: 25852657 Free PMC article.
-
Archaeal bundling pili of Pyrobaculum calidifontis reveal similarities between archaeal and bacterial biofilms.Proc Natl Acad Sci U S A. 2022 Jun 28;119(26):e2207037119. doi: 10.1073/pnas.2207037119. Epub 2022 Jun 21. Proc Natl Acad Sci U S A. 2022. PMID: 35727984 Free PMC article.
-
Cryo-EM of bacterial pili and archaeal flagellar filaments.Curr Opin Struct Biol. 2017 Oct;46:31-37. doi: 10.1016/j.sbi.2017.05.012. Epub 2017 Jun 10. Curr Opin Struct Biol. 2017. PMID: 28609682 Free PMC article. Review.
-
Diversity, assembly and regulation of archaeal type IV pili-like and non-type-IV pili-like surface structures.Res Microbiol. 2012 Nov-Dec;163(9-10):630-44. doi: 10.1016/j.resmic.2012.10.024. Epub 2012 Nov 9. Res Microbiol. 2012. PMID: 23146836 Review.
Cited by
-
Towards a molecular picture of the archaeal cell surface.Nat Commun. 2024 Nov 29;15(1):10401. doi: 10.1038/s41467-024-53986-9. Nat Commun. 2024. PMID: 39614099 Free PMC article.
-
Cell surface architecture of the cultivated DPANN archaeon Nanobdella aerobiophila.J Bacteriol. 2024 Feb 22;206(2):e0035123. doi: 10.1128/jb.00351-23. Epub 2024 Jan 30. J Bacteriol. 2024. PMID: 38289045 Free PMC article.
-
The assembly platform FimD is required to obtain the most stable quaternary structure of type 1 pili.Nat Commun. 2024 Apr 8;15(1):3032. doi: 10.1038/s41467-024-47212-9. Nat Commun. 2024. PMID: 38589417 Free PMC article.
-
Donor Strand Complementation and Calcium Ion Coordination Drive the Chaperone-free Polymerization of Archaeal Cannulae.bioRxiv [Preprint]. 2024 Dec 30:2024.12.30.630787. doi: 10.1101/2024.12.30.630787. bioRxiv. 2024. PMID: 39803462 Free PMC article. Preprint.
-
Archaeal virus entry and egress.Microlife. 2024 Jan 3;5:uqad048. doi: 10.1093/femsml/uqad048. eCollection 2024. Microlife. 2024. PMID: 38234448 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

