Strong pathogen competition in neonatal gut colonisation
- PMID: 36456554
- PMCID: PMC9715557
- DOI: 10.1038/s41467-022-35178-5
Strong pathogen competition in neonatal gut colonisation
Abstract
Opportunistic bacterial pathogen species and their strains that colonise the human gut are generally understood to compete against both each other and the commensal species colonising this ecosystem. Currently we are lacking a population-wide quantification of strain-level colonisation dynamics and the relationship of colonisation potential to prevalence in disease, and how ecological factors might be modulating these. Here, using a combination of latest high-resolution metagenomics and strain-level genomic epidemiology methods we performed a characterisation of the competition and colonisation dynamics for a longitudinal cohort of neonatal gut microbiomes. We found strong inter- and intra-species competition dynamics in the gut colonisation process, but also a number of synergistic relationships among several species belonging to genus Klebsiella, which includes the prominent human pathogen Klebsiella pneumoniae. No evidence of preferential colonisation by hospital-adapted pathogen lineages in either vaginal or caesarean section birth groups was detected. Our analysis further enabled unbiased assessment of strain-level colonisation potential of extra-intestinal pathogenic Escherichia coli (ExPEC) in comparison with their propensity to cause bloodstream infections. Our study highlights the importance of systematic surveillance of bacterial gut pathogens, not only from disease but also from carriage state, to better inform therapies and preventive medicine in the future.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
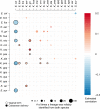

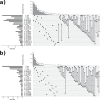
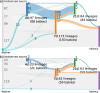


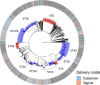
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

