Safety and efficacy of HSP90 inhibitor ganetespib for neoadjuvant treatment of stage II/III breast cancer
- PMID: 36456573
- PMCID: PMC9715670
- DOI: 10.1038/s41523-022-00493-z
Safety and efficacy of HSP90 inhibitor ganetespib for neoadjuvant treatment of stage II/III breast cancer
Abstract
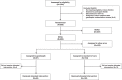
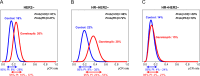
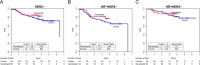
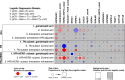
HSP90 inhibitors destabilize oncoproteins associated with cell cycle, angiogenesis, RAS-MAPK activity, histone modification, kinases and growth factors. We evaluated the HSP90-inhibitor ganetespib in combination with standard chemotherapy in patients with high-risk early-stage breast cancer. I-SPY2 is a multicenter, phase II adaptively randomized neoadjuvant (NAC) clinical trial enrolling patients with stage II-III breast cancer with tumors 2.5 cm or larger on the basis of hormone receptors (HR), HER2 and Mammaprint status. Multiple novel investigational agents plus standard chemotherapy are evaluated in parallel for the primary endpoint of pathologic complete response (pCR). Patients with HER2-negative breast cancer were eligible for randomization to ganetespib from October 2014 to October 2015. Of 233 women included in the final analysis, 140 were randomized to the standard NAC control; 93 were randomized to receive 150 mg/m2 ganetespib every 3 weeks with weekly paclitaxel over 12 weeks, followed by AC. Arms were balanced for hormone receptor status (51-52% HR-positive). Ganetespib did not graduate in any of the biomarker signatures studied before reaching maximum enrollment. Final estimated pCR rates were 26% vs. 18% HER2-negative, 38% vs. 22% HR-negative/HER2-negative, and 15% vs. 14% HR-positive/HER2-negative for ganetespib vs control, respectively. The predicted probability of success in phase 3 testing was 47% HER2-negative, 72% HR-negative/HER2-negative, and 19% HR-positive/HER2-negative. Ganetespib added to standard therapy is unlikely to yield substantially higher pCR rates in HER2-negative breast cancer compared to standard NAC, and neither HSP90 pathway nor replicative stress expression markers predicted response. HSP90 inhibitors remain of limited clinical interest in breast cancer, potentially in other clinical settings such as HER2-positive disease or in combination with anti-PD1 neoadjuvant chemotherapy in triple negative breast cancer.Trial registration: www.clinicaltrials.gov/ct2/show/NCT01042379.
© 2022. The Author(s).
Conflict of interest statement
Julie E. Lang declares no competing non-financial interests, except the following competing financial interests: received a research grant from ANGLE Parsortix, and the University of Southern California; and received honoraria as part of the Genomic Health’s speakers’ bureau. A. Forero-Torres declares no competing non-financial interests, except the following competing financial interests: became a Seattle Genetics employee in 2018; and holds stock option from this employment. Doug Yee declares no competing non-financial interests, except the following competing financial interests: received research support from Quantum Leap Healthcare Collaborative, Fusion Pharmaceutical, the National Cancer Institute, and Boehringer Ingleheim; received consulting fees from Martell Diagnositics; received travel support from the University of Texas Health Science Center at San Antonio; and received support from Akston Biosciences. Christina Yau declares no competing non-financial interests, except the following competing financial interests: received research support from Quantum Leap Healthcare Collaborative and the National Cancer Institute; and received travel support from Quantum Leap Healthcare Collaborative. Barbara Parker declares no competing non-financial interests, except the following competing financial interests: received research support from Pfizer, Novartis, Glaxo Smith Kline, Genentech/Roche, Oncternal Therapeutics Inc; received consulting fees from Dare Biosciences; and serves as an advisor on the San Diego County Susan G. Komen Advisory Board. A. Jo Chien declares no competing non-financial interests, except the following competing financial interests: received institutional research support from Seagen, Merck, Amgen and Puma Biotechnology. Barbara B. Haley declares no competing non-financial interests, except the following competing financial interests: received institutional research funding from Pfizer, Lilly, Daiichi Sankyo, Roche, Puma, Astra Zeneca and Sanofi. Judy C. Boughey declares no competing non-financial interests, except the following competing financial interest: received research support from Eli Lilly. Claudine Isaacs declares no competing non-financial interests, except the following competing financial interests: received consulting fees from Seattle Genetics, Genentech, AstraZeneca, Novartis, PUMA, Pfizer, Gilead, ION Pharma, Sanofi, and Esai; received honoraria from Genentech; received research support from Quantum Leap Healthcare Collaborative; received grants from Tesaro/Glaxo Smith Kline, Seattle Genetics, Pfizer, AstraZeneca, Bristol Myers Squibb, Genentech, and Novartis; received royalties from Wolters Kluwer; participated on a Data Safety Monitoring Board for Novartis; participated on an Advisory Board for Genentech, PUMA, Seattle Genetics, AstraZeneca, Novartis, Pfizer, Eisai, Sanofi, ION, and Gilead; and serves as Medical Director for the SideOut Foundation. Hyo S. Han declares no competing non-financial interests, except the following competing financial interests: reports institutional research funding from Abbvie, Arvinas, Glaxo Smith Kline, G1 Therapeutics, Quantum Leap Healthcare Collaborative, Marker, Pfizer, Zymeworks, Celcuity, and the Department of Defense; received honoraria from Lilly’s speaker’s bureau; and reports participation on an Advisory Board for Novartis, AstraZeneca, and Gilead. Rita Nanda declares no competing non-financial interests, except the following competing financial interests: received research support from Arvinas, AstraZeneca, Celgene, Corcept Therapeutics, Genentech/Roche, Immunomedics/Gilead, Merck, OBI Pharm, Inc., Odonate Therapeutics, OncoSec, Pfizer, Taiho, Relay, Sun Pharma and Seattle Genetics; and has received consulting fees from AstraZeneca, BeyondSpring, Fujifilm, Gilead, Infinity, iTeos, Merck, OBI, Oncosec, and Seagen. Erica Stringer-Reasor declares no competing non-financial interests, except the following competing financial interests: received research support and/or consulting fees from Lilly, Immunomedics, Seagen, and Mylan; received honoraria from Lilly; and received grants from the Susan G. Koman Foundation, V. Foundation, and BCRFA. Bonnie Joe declares no competing non-financial interests, except the following competing financial interests: received author royalties from UpToDate; received institutional research funding from Kheiron Medical Technologies; and honoraria from World Class CME. Emanuel F. Petricoin declares no competing non-financial interests, except the following competing financial interests: reports leadership roles with Perthera, Theralink Technologies, and Ceres Nanosciences; stock or other ownership interests in Perthera, Theralink Technologies, and Ceres Nanosciences; and consulting or advisory roles with Perthera, Ceres Nanosciences, Theralink Technologies, and Peytant Solutions; reports patents, royalties, other intellectual property from National Institutes of Health patents licensing fee distribution/royalty, co-inventor on filed George Mason University–assigned patents related to phosphorylated HER2 and EGFR response predictors for HER family-directed therapeutics, as such can receive royalties and licensing distribution on any licensed IP. Julia D. Wulfkuhle declares no competing non-financial interests, except the following competing financial interests: owns stock in Theralink Technologies. Donald Berry and Scott Berry declare no competing non-financial interests, except the following competing financial interests: are co-owners of Berry Consultants, LLC, a company that designs adaptive Bayesian clinical trials (including I-SPY 2) for pharmaceutical and medical device companies, NIH cooperative groups, patient advocacy groups, and international consortia. Hope Rugo declares no competing non-financial interests, except the following competing financial interests: reports institutional research support from Pfizer, Merck, Novartis, Lilly, Daiichi, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala, Astellas, and Gilead; received honoraria from Puma Biotechnology, Samsung, Chugai, Blueprint and NAPO; and received travel support from GE Healthcare. W. Fraser Symmans declares no competing non-financial interests, except the following competing financial interests: is a co-founder with equity in Delphi Diagnostics that licensed intellectual property; is co-inventor of an issued patent for the algorithm to calculate residual cancer burden that is freely available on the internet; holds publicly traded shares in the following companies: IONIS Pharmaceuticals, Eiger Biopharmaceuticals; received institutional funding from the National Cancer Institute, The Cancer Prevention and Research Institute of Texas, Pfizer and the Breast Cancer Research Foundation. Laura J. van ‘t Veer declares no competing non-financial interests, except the following competing financial interests: is a part-time employee and stockholder in Agendia N.V. Angela DeMichele declares the following financial interests: received institutional research support from Novartis, Pfizer, Genentech, Calithera and Inivata; and honoraria from the NCI Breast Cancer Steering Committee; declares competing financial interests but the following competing non-financial interests: unpaid leadership roles with the American Society of Clinical Oncology, AACR San Antonio Breast Cancer Symposium, ECOG/ACRIN Cooperative Group. Laura Esserman declares competing financial interests but the following competing non-financial interests: is an unpaid member of the board of directors of Quantum Leap Healthcare Collaborative (QLHC); declares the following competing financial interests: received grant support from QLHC for the I-SPY2 Trial. Nola Hylton declares no competing non-financial interests, except the following competing financial interests: reports instutional grants from the NIH NCI (U01 CA225427, R01 CA132870, P01 CA210961). Jane Perlmutter declares no competing non-financial interests, except the following competing financial interests: participation on a Data Safety Monitoring Board for QuantumLeap, and on an Advisory Board for VIVLI, University of Wisconsin SPORE, and PCORI; and received honoraria from Methods in Clinical Research. Denise Wolf declares no competing non-financial interests, except the following competing financial interests: reports institutional research support from the National Cancer Institute and Quantum Leap Healthcare Collaborative. Gillian Hirst declares no competing non-financial interests, except the following competing financial interests: owns stock in Moderna, Exact Sciences, Gilead and Nanostring. Lamora Brown-Swigart declares no competing non-financial interests, except the following competing financial interests: institutional research support from the NIH and Quantum Leap Healthcare Collaborative. Minetta Liu declares no competing non-financial interests, except the following competing financial interests: reports institutional research support from Eisai, Exact Sciences, Genentech, Genomic Health, GRAIL, Menarini Silicon Biosystems, Merck, Novartis, Seattle Genetics and Tesaro; received travel support from AstraZeneca, Genomic Health and Ionis; and served on an Advisory Board for AstraZeneca, Celgene, Roche/Genentech, Genomic Health, GRAIL, Ionis, Merck, Pfizer, Seattle Genetics and Syndax. Kathy Albain declares no competing non-financial interests, except the following competing financial interests: received research support from Quantum Leap Healthcare Collaborative, Merck, Seattle Genetics, Amgen, Genentech-Roche; received institutional research support from Seattle Genetics, Daiichi-Sankyo, and AstraZeneca; and served on an Advisory Board for Genomic Health/Exact Sciences, Genentech-Roche, and Seattle Genetics/Axio. Rashmi Murthy declares no competing non-financial interests, except the following competing financial interests: received research funding from Seattle Genetics, Pfizer, AstraZeneca, EMD Serono, Genentech/Roche, and Daiichi-Sankyo; received consulting fees from Sanofi, AstraZeneca, Pfizer, Genentech/Roche, Novartis, Seattle Geneetics, and Puma Technology; received honoraria from Puma Biotechnology, Seattle Genetics, Genentech, Novartis and AstraZeneca; and has received travel support from Genentech and Seattle Genetics. Meredith Buxton declares no competing non-financial interests, except the following competing financial interests: received research funding from Synta Pharmaceuticals, Quintiles, the Safeway Foundation, William K. Bowes Foundation, FNIH and the Breast Cancer Research Foundation; has received grants from Bayer Pharmaceuticals, Kazia Therapeautics, Kintara Therapeutics, Amgen, ERASCA Inc. Ashish Sunil declares no competing non-financial interests, except the following competing financial interests: is an employee of Berry Consultants LLC. John Park, Anne Wallace, Erin Ellis, Heather Beckwith, Anthony Elias, Rachel Yung, Amy Clark, Qamar Khan, Kristen Edmiston, Elissa Price, Julia Clennell, Smita Asare, Amy Wilson, Ruby Singhrao, Adam Asare, Jeffrey Matthews, and Michelle Melisko declare no competing financial or non-financial interests.
Figures
References
-
- Beliakoff J, Whitesell L. Hsp90: an emerging target for breast cancer therapy. Anti-cancer Drug. 2004;15:651–662. doi: 10.1097/01.cad.0000136876.11928.be. - DOI - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

