Conditioned medium of mesenchymal stem cells pretreated with H2O2 promotes intestinal mucosal repair in acute experimental colitis
- PMID: 36456585
- PMCID: PMC9715703
- DOI: 10.1038/s41598-022-24493-y
Conditioned medium of mesenchymal stem cells pretreated with H2O2 promotes intestinal mucosal repair in acute experimental colitis
Abstract
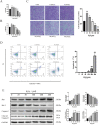
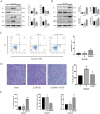
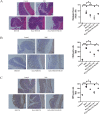
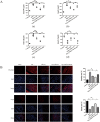
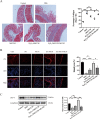
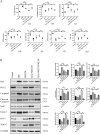
Mesenchymal stem cells (MSCs) are a new therapeutic strategy for inflammatory bowel disease (IBD), and their efficacy has been widely recognized. However, there are still some challenges in cell therapy, including stable cell passage, laboratory conditions for cell culture, high-cost burden, and poor transplantation. The conditioned medium (CM) of MSCs is considered be an excellent alternative to cell transplantation, but the paracrine group in MSC-CM is limited in variety and low in concentration, which cannot meet the therapeutic needs of injured tissues and needs to be optimized. Pretreatment with low concentration of hydrogen peroxide (H2O2) can not only protect cells from oxidative damage, but also play a role similar to growth factors and regulate the physiological function of stem cells, to obtain an improved conditioned medium. To determine the optimal protocol for pretreatment of MSCs with H2O2, and to study the efficacy and potential mechanism of MSC-CM pretreated with H2O2 on Dextran Sulfate Sodium (DSS)-induced acute experimental colitis. MSCs were exposed to different concentrations of H2O2, and the optimal H2O2 pretreatment conditions were determined by evaluating their critical cell functional properties. H2O2-pretreated MSC-CM was transplanted into experimental mouse colitis by enema at 2, 4, and 6 days in modeling, and the changes of colonic tissue structure, the levels of inflammation and oxidative stress, the molecular changes of Nrf2/Keap1/ARE axis, and the related indicators of apoptosis in colonic epithelial cells were observed in each group. In vitro, Pretreated MSCs with 25 μM H2O2 significantly enhanced cell proliferation, migration, and survival, but had no effect on apoptosis. In vivo, MSC-CM treatment decreased apoptosis and extracellular matrix deposition, and maintained the mechanical barrier and permeability of colonic epithelial cells in experimental mouse colitis. Mechanistically, H2O2-pretreated MSC-CM against reactive oxygen species (ROS) production and MDA generation, accompanied by increases in components of the enzymatic antioxidant system includes SOD, CAT, GSH-PX, and T-AOC, which is through the up-regulation of the Nrf2, HO-1, and NQO-1 antioxidant genes. Our data confirmed that 25 μM H2O2 pretreated MSC-CM treatment could effectively improve intestinal mucosal repair in experimental colitis, which may be achieved by activating Nrf2/Keap1/ARE pathway.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Mesenchymal Stem Cells Promote Intestinal Mucosal Repair by Positively Regulating the Nrf2/Keap1/ARE Signaling Pathway in Acute Experimental Colitis.Dig Dis Sci. 2023 May;68(5):1835-1846. doi: 10.1007/s10620-022-07722-2. Epub 2022 Dec 2. Dig Dis Sci. 2023. PMID: 36459293
-
Conditioned mesenchymal stem cells produce pleiotropic gut trophic factors.J Gastroenterol. 2014 Feb;49(2):270-82. doi: 10.1007/s00535-013-0901-3. Epub 2013 Nov 12. J Gastroenterol. 2014. PMID: 24217964
-
Dissecting molecular mechanisms underlying H2O2-induced apoptosis of mouse bone marrow mesenchymal stem cell: role of Mst1 inhibition.Stem Cell Res Ther. 2020 Dec 9;11(1):526. doi: 10.1186/s13287-020-02041-7. Stem Cell Res Ther. 2020. PMID: 33298178 Free PMC article.
-
Potential of mesenchymal stem cell-derived conditioned medium/secretome as a therapeutic option for ocular diseases.Regen Med. 2023 Oct;18(10):795-807. doi: 10.2217/rme-2023-0089. Epub 2023 Sep 13. Regen Med. 2023. PMID: 37702008 Review.
-
Female Reproductive Aging and Oxidative Stress: Mesenchymal Stem Cell Conditioned Medium as a Promising Antioxidant.Int J Mol Sci. 2023 Mar 6;24(5):5053. doi: 10.3390/ijms24055053. Int J Mol Sci. 2023. PMID: 36902477 Free PMC article. Review.
Cited by
-
Therapeutic Prospects of Mesenchymal Stem Cell and Their Derived Exosomes in the Regulation of the Gut Microbiota in Inflammatory Bowel Disease.Pharmaceuticals (Basel). 2024 May 9;17(5):607. doi: 10.3390/ph17050607. Pharmaceuticals (Basel). 2024. PMID: 38794176 Free PMC article. Review.
-
Therapeutic potential of mesenchymal stem cell-derived extracellular vesicles: A focus on inflammatory bowel disease.Clin Transl Med. 2024 Nov;14(11):e70075. doi: 10.1002/ctm2.70075. Clin Transl Med. 2024. PMID: 39488745 Free PMC article. Review.
-
The role of mesenchymal stem cells in cancer and prospects for their use in cancer therapeutics.MedComm (2020). 2024 Jul 28;5(8):e663. doi: 10.1002/mco2.663. eCollection 2024 Aug. MedComm (2020). 2024. PMID: 39070181 Free PMC article. Review.
-
Mechanism of cell death and its application in the repair of inflammatory bowel disease by mesenchymal stem cells.Front Immunol. 2025 Jun 4;16:1597462. doi: 10.3389/fimmu.2025.1597462. eCollection 2025. Front Immunol. 2025. PMID: 40534879 Free PMC article. Review.
-
Conditioned medium from adipose mesenchymal stromal cells stimulated with pituitary adenylate cyclase-activating polypeptide (PACAP) mitigates RINm5F pancreatic β-cell dysfunction.Mol Biol Rep. 2025 Jul 9;52(1):689. doi: 10.1007/s11033-025-10717-7. Mol Biol Rep. 2025. PMID: 40632340 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

