Analysis of genome-wide knockout mouse database identifies candidate ciliopathy genes
- PMID: 36456625
- PMCID: PMC9715561
- DOI: 10.1038/s41598-022-19710-7
Analysis of genome-wide knockout mouse database identifies candidate ciliopathy genes
Abstract
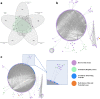
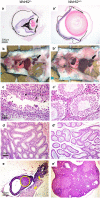
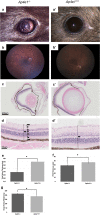
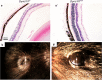
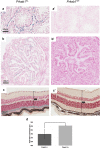
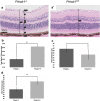
We searched a database of single-gene knockout (KO) mice produced by the International Mouse Phenotyping Consortium (IMPC) to identify candidate ciliopathy genes. We first screened for phenotypes in mouse lines with both ocular and renal or reproductive trait abnormalities. The STRING protein interaction tool was used to identify interactions between known cilia gene products and those encoded by the genes in individual knockout mouse strains in order to generate a list of "candidate ciliopathy genes." From this list, 32 genes encoded proteins predicted to interact with known ciliopathy proteins. Of these, 25 had no previously described roles in ciliary pathobiology. Histological and morphological evidence of phenotypes found in ciliopathies in knockout mouse lines are presented as examples (genes Abi2, Wdr62, Ap4e1, Dync1li1, and Prkab1). Phenotyping data and descriptions generated on IMPC mouse line are useful for mechanistic studies, target discovery, rare disease diagnosis, and preclinical therapeutic development trials. Here we demonstrate the effective use of the IMPC phenotype data to uncover genes with no previous role in ciliary biology, which may be clinically relevant for identification of novel disease genes implicated in ciliopathies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

