Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis
- PMID: 36456739
- PMCID: PMC9747617
- DOI: 10.1038/s41590-022-01354-4
Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis
Erratum in
-
Author Correction: Parenteral BCG vaccine induces lung-resident memory macrophages and trained immunity via the gut-lung axis.Nat Immunol. 2023 Jun;24(6):1049. doi: 10.1038/s41590-023-01525-x. Nat Immunol. 2023. PMID: 37161093 Free PMC article. No abstract available.
Abstract
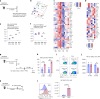
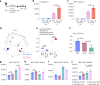
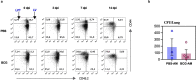
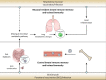
Aside from centrally induced trained immunity in the bone marrow (BM) and peripheral blood by parenteral vaccination or infection, evidence indicates that mucosal-resident innate immune memory can develop via a local inflammatory pathway following mucosal exposure. However, whether mucosal-resident innate memory results from integrating distally generated immunological signals following parenteral vaccination/infection is unclear. Here we show that subcutaneous Bacillus Calmette-Guérin (BCG) vaccination can induce memory alveolar macrophages (AMs) and trained immunity in the lung. Although parenteral BCG vaccination trains BM progenitors and circulating monocytes, induction of memory AMs is independent of circulating monocytes. Rather, parenteral BCG vaccination, via mycobacterial dissemination, causes a time-dependent alteration in the intestinal microbiome, barrier function and microbial metabolites, and subsequent changes in circulating and lung metabolites, leading to the induction of memory macrophages and trained immunity in the lung. These data identify an intestinal microbiota-mediated pathway for innate immune memory development at distal mucosal tissues and have implications for the development of next-generation vaccine strategies against respiratory pathogens.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures














Comment in
-
Intestinal microbe-derived metabolites instruct macrophages in the lungs.Nat Immunol. 2022 Dec;23(12):1662-1664. doi: 10.1038/s41590-022-01358-0. Nat Immunol. 2022. PMID: 36456738 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

