DCC/netrin-1 regulates cell death in oligodendrocytes after brain injury
- PMID: 36456775
- PMCID: PMC9950151
- DOI: 10.1038/s41418-022-01091-z
DCC/netrin-1 regulates cell death in oligodendrocytes after brain injury
Abstract
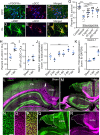
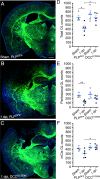
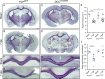
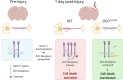
Hallmark pathological features of brain trauma are axonal degeneration and demyelination because myelin-producing oligodendrocytes (OLs) are particularly vulnerable to injury-induced death signals. To reveal mechanisms responsible for this OL loss, we examined a novel class of "death receptors" called dependence receptors (DepRs). DepRs initiate pro-death signals in the absence of their respective ligand(s), yet little is known about their role after injury. Here, we investigated whether the deleted in colorectal cancer (DCC) DepR contributes to OL loss after brain injury. We found that administration of its netrin-1 ligand is sufficient to block OL cell death. We also show that upon acute injury, DCC is upregulated while netrin-1 is downregulated in perilesional tissues. Moreover, after genetically silencing pro-death activity using DCCD1290N mutant mice, we observed greater OL survival, greater myelin integrity, and improved motor function. Our findings uncover a novel role for the netrin-1/DCC pathway in regulating OL loss in the traumatically injured brain.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. 2019;130:1080–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

