Efficacy of supermarket and web-based interventions for improving dietary quality: a randomized, controlled trial
- PMID: 36456831
- PMCID: PMC9800276
- DOI: 10.1038/s41591-022-02077-7
Efficacy of supermarket and web-based interventions for improving dietary quality: a randomized, controlled trial
Abstract
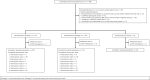
Dietary interventions may best be delivered at supermarkets, which offer convenience, accessibility, full food inventories and, increasingly, in-store registered dietitians, online shopping and delivery services. In collaboration with a large retail supermarket chain, we conducted a multisite supermarket and web-based intervention targeting nutrition trial (no. NCT03895580), randomizing participants (n = 247 (139 women and 108 men)) 2:2:1 to two levels of dietary education (Strategy 1 and Strategy 2) or an enhanced control group that included educational components beyond the routine standard of care. Both Strategies 1 and 2 included individualized, in-person, dietitian-led, purchasing data-guided interventions. Strategy 2 also included online tools for shopping, home delivery, selection of healthier purchases, meal planning and healthy recipes. The primary endpoint was change in dietary approaches to stop hypertension (DASH) score (a measure of adherence to the DASH diet) from baseline to 3 months. The primary endpoint was met because, at 3 months, the DASH score increased by 4.7 more for the combined Strategy 1 and Strategy 2 groups than for the control group (95% confidence interval (CI) (0.9, 8.5), P = 0.02). In a prespecified hierarchical test, at 3 months, DASH score increased by 3.8 more for the Strategy 2 group than for the Strategy 1 group (95% CI (0.8, 6.)9, P = 0.01). This trial demonstrates the efficacy of data-guided, supermarket-based, dietary interventions and modern online shopping tools in improving dietary quality in a free-living, community-based population. The trial also demonstrates the opportunity for academic investigators to collaborate with retailers to design and rigorously test comprehensive healthcare interventions.
© 2022. The Author(s).
Conflict of interest statement
D.L.S. and S.C.C. received funding to support their effort on this study from The Kroger Company. D.L.S. also discloses the following relationships: Consultant, Sanofi; CEO, High Enroll, LLC. D.L.B. discloses the following relationships: Advisory Board, AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences and Stasys; Board of Directors, AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), Society of Cardiovascular Patient Care, TobeSoft; Chair, Inaugural Chair, American Heart Association Quality Oversight Committee; Consultant, Broadview Ventures; Data Monitoring Committees, Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria, American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee and USA national coleader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); other, Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); patent, Sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither I nor Brigham and Women’s Hospital receive any income from this patent.); research funding, Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); site coinvestigator, Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; trustee, American College of Cardiology; unfunded research, FlowCo, Takeda. S.C.C., R.N.H., B.E.S., M.H.E., S.S.S., M.F. and E.C.K. declare no competing interests.
Figures
Comment in
-
Fixing America's eating habits with effective stakeholder collaborations.Nat Med. 2022 Dec;28(12):2469-2470. doi: 10.1038/s41591-022-02111-8. Nat Med. 2022. PMID: 36471038 No abstract available.
References
-
- Tsao C. W. et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation10.1161/CIR.0000000000001052 (2022). - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous