Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: Results from a 52-Week Open-Label Phase IV Clinical Trial (DIMESKIN 1)
- PMID: 36456890
- PMCID: PMC9823187
- DOI: 10.1007/s13555-022-00863-2
Efficacy and Safety of Dimethyl Fumarate in Patients with Moderate-to-Severe Plaque Psoriasis: Results from a 52-Week Open-Label Phase IV Clinical Trial (DIMESKIN 1)
Abstract
Introduction: Although dimethyl fumarate (DMF) has been approved since 2017 for treatment of moderate-to-severe plaque psoriasis, limited data on its safety and efficacy are available in clinical practice. The objective was to assess the efficacy and safety of DMF in patients with moderate-to-severe plaque psoriasis through 52 weeks in conditions close to real clinical practice.
Methods: DIMESKIN 1 was a 52-week, open-label, phase IV clinical trial conducted at 36 Spanish sites. Adults with diagnosis of moderate-to-severe plaque psoriasis, treated with DMF as per its summary of product characteristics and with ≥ 1 post-baseline Psoriasis Area and Severity Index (PASI) value were included [intention-to-treat (ITT) population]. Efficacy analyses were performed for ITT population and are based on multiple imputation.
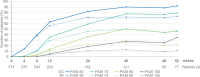
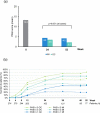
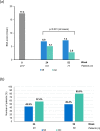
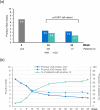
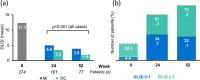
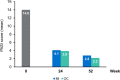
Results: Overall, 282 and 274 patients were included in the safety and ITT populations, respectively. At week 24, 46.0%/24.8%/10.9% of patients achieved PASI 75/90/100 response, respectively. At week 52, these percentages were 46.0%/21.9%/10.9%, respectively. Mean body surface area affected decreased from 17.4% to 6.9%/7.3% after 24/52 weeks (p < 0.001, both). A total of 42.9%/49.4% of patients had a Physician's Global Assessment 0-1 at week 24/52, respectively. Mean pruritus visual analogue scale (VAS) significantly decreased after 24 and 52 weeks (p < 0.001, both), with 56.5% and 67.6% of patients, respectively, rating a pruritus VAS < 3. At week 24/52, 61.3%/73.4% patients had a Dermatology Life Quality Index (DLQI) ≤ 5 and 34.7%/32.1% had a DLQI 0-1. The most frequent adverse events were gastrointestinal disorders (mainly diarrhea/abdominal pain in 50.0%/35.1% of patients, respectively), flushing (28.0%), and lymphopenia (31.2%), mostly mild/moderate.
Conclusions: DMF significantly improves main severity and extension indexes and rates, as well as patient-reported outcomes such as pruritus and quality of life in patients with moderate-to-severe psoriasis after 24 weeks of treatment. These improvements are sustained through 52 weeks. The safety profile of DMF is similar to that previously described for fumarates.
Eudract number: 2017-00136840.
Keywords: Clinical practice; Dimethyl fumarate; Efficacy; Psoriasis; Safety.
© 2022. The Author(s).
Figures
References
-
- Enamandram M, Kimball AB. Psoriasis epidemiology: the interplay of genes and the environment. J Invest Dermatol. 2013;133(2):287–289. - PubMed
-
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735–1741. - PubMed
-
- Candia R, Ruiz A, Torres-Robles R, Chávez-Tapia N, Méndez-Sánchez N, Arrese M. Risk of non-alcoholic fatty liver disease in patients with psoriasis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2015;29(4):656–662. - PubMed
LinkOut - more resources
Full Text Sources

