Outcomes in participants with failure of initial antibacterial therapy for hospital-acquired/ventilator-associated bacterial pneumonia prior to enrollment in the randomized, controlled phase 3 ASPECT-NP trial of ceftolozane/tazobactam versus meropenem
- PMID: 36457059
- PMCID: PMC9714015
- DOI: 10.1186/s13054-022-04192-w
Outcomes in participants with failure of initial antibacterial therapy for hospital-acquired/ventilator-associated bacterial pneumonia prior to enrollment in the randomized, controlled phase 3 ASPECT-NP trial of ceftolozane/tazobactam versus meropenem
Abstract
Background: Ceftolozane/tazobactam, a combination antibacterial agent comprising an anti-pseudomonal cephalosporin and β-lactamase inhibitor, is approved for the treatment of hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP) in adults. Participants in the ASPECT-NP trial received ceftolozane/tazobactam (3 g [2 g ceftolozane/1 g tazobactam] every 8 h) or meropenem (1 g every 8 h). Participants failing prior antibacterial therapy for the current HABP/VABP episode at study entry had lower 28-day all-cause mortality (ACM) rates with ceftolozane/tazobactam versus meropenem treatment. Here, we report a post hoc analysis examining this result.
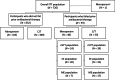
Methods: The phase 3, randomized, controlled, double-blind, multicenter, noninferiority trial compared ceftolozane/tazobactam versus meropenem for treatment of adults with ventilated HABP/VABP; eligibility included those failing prior antibacterial therapy for the current HABP/VABP episode at study entry. The primary and key secondary endpoints were 28-day ACM and clinical response at test of cure (TOC), respectively. Participants who were failing prior therapy were a prospectively defined subgroup; however, subgroup analyses were not designed for noninferiority testing. The 95% CIs for treatment differences were calculated as unstratified Newcombe CIs. Post hoc analyses were performed using multivariable logistic regression analysis to determine the impact of baseline characteristics and treatment on clinical outcomes in the subgroup who were failing prior antibacterial therapy.
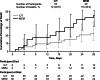
Results: In the ASPECT-NP trial, 12.8% of participants (93/726; ceftolozane/tazobactam, n = 53; meropenem, n = 40) were failing prior antibacterial therapy at study entry. In this subgroup, 28-day ACM was higher in participants who received meropenem versus ceftolozane/tazobactam (18/40 [45.0%] vs 12/53 [22.6%]; percentage difference [95% CI]: 22.4% [3.1 to 40.1]). Rates of clinical response at TOC were 26/53 [49.1%] for ceftolozane/tazobactam versus 15/40 [37.5%] for meropenem (percentage difference [95% CI]: 11.6% [- 8.6 to 30.2]). Multivariable regression analysis determined concomitant vasopressor use and treatment with meropenem were significant factors associated with risk of 28-day ACM. Adjusting for vasopressor use, the risk of dying after treatment with ceftolozane/tazobactam was approximately one-fourth the risk of dying after treatment with meropenem.
Conclusions: This post hoc analysis further supports the previously demonstrated lower ACM rate for ceftolozane/tazobactam versus meropenem among participants who were failing prior therapy, despite the lack of significant differences in clinical cure rates.
Clinicaltrials: gov registration NCT02070757 . Registered February 25, 2014, clinicaltrials.gov/ct2/show/NCT02070757 .
Keywords: All-cause mortality; Clinical response; Failing prior antibacterial therapy; HABP; Mechanical ventilation; Multivariable analysis; Nosocomial pneumonia; Refractory; VABP.
© 2022. The Author(s).
Conflict of interest statement
MHK is a consultant for MSD and Shionogi and his efforts are supported by the Barnes-Jewish Hospital Foundation. JFT has received institutional research support from MSD. IML has received institutional research support from MSD. RGW has received institutional research support and consultancy fees from MSD. JAH, EHJ, BY, and CJB are employees of MSD, and own stock and/or hold stock options in Merck & Co., Inc., Rahway, NJ, USA.
Figures
Similar articles
-
Perspectives on the use of ceftolozane/tazobactam: a review of clinical trial data and real-world evidence.Future Microbiol. 2024;19(6):465-480. doi: 10.2217/fmb-2023-0197. Epub 2024 Jan 22. Future Microbiol. 2024. PMID: 38252038 Free PMC article. Review.
-
Ceftolozane/tazobactam versus meropenem in patients with ventilated hospital-acquired bacterial pneumonia: subset analysis of the ASPECT-NP randomized, controlled phase 3 trial.Crit Care. 2021 Aug 11;25(1):290. doi: 10.1186/s13054-021-03694-3. Crit Care. 2021. PMID: 34380538 Free PMC article. Clinical Trial.
-
Ceftolozane-tazobactam versus meropenem for treatment of nosocomial pneumonia (ASPECT-NP): a randomised, controlled, double-blind, phase 3, non-inferiority trial.Lancet Infect Dis. 2019 Dec;19(12):1299-1311. doi: 10.1016/S1473-3099(19)30403-7. Epub 2019 Sep 25. Lancet Infect Dis. 2019. PMID: 31563344 Clinical Trial.
-
Ceftolozane/tazobactam for hospital-acquired/ventilator-associated bacterial pneumonia due to ESBL-producing Enterobacterales: a subgroup analysis of the ASPECT-NP clinical trial.J Antimicrob Chemother. 2022 Aug 25;77(9):2522-2531. doi: 10.1093/jac/dkac184. J Antimicrob Chemother. 2022. PMID: 35781341 Clinical Trial.
-
Ceftolozane-tazobactam in nosocomial pneumonia.Rev Esp Quimioter. 2022 Apr;35 Suppl 1(Suppl 1):35-39. doi: 10.37201/req/s01.08.2022. Epub 2022 Apr 22. Rev Esp Quimioter. 2022. PMID: 35488823 Free PMC article. Review.
Cited by
-
Clinical efficacy, safety and pharmacokinetics of novel β-lactam/β-lactamase inhibitor combinations: a systematic review.JAC Antimicrob Resist. 2025 Jun 19;7(3):dlaf096. doi: 10.1093/jacamr/dlaf096. eCollection 2025 Jun. JAC Antimicrob Resist. 2025. PMID: 40583996 Free PMC article. Review.
-
Resistance in Pseudomonas aeruginosa: A Narrative Review of Antibiogram Interpretation and Emerging Treatments.Antibiotics (Basel). 2023 Nov 12;12(11):1621. doi: 10.3390/antibiotics12111621. Antibiotics (Basel). 2023. PMID: 37998823 Free PMC article. Review.
-
Perspectives on the use of ceftolozane/tazobactam: a review of clinical trial data and real-world evidence.Future Microbiol. 2024;19(6):465-480. doi: 10.2217/fmb-2023-0197. Epub 2024 Jan 22. Future Microbiol. 2024. PMID: 38252038 Free PMC article. Review.
-
Novel Antibiotics for Gram-Negative Nosocomial Pneumonia.Antibiotics (Basel). 2024 Jul 5;13(7):629. doi: 10.3390/antibiotics13070629. Antibiotics (Basel). 2024. PMID: 39061311 Free PMC article. Review.
-
The synergistic effect between phages and Ceftolozane/Tazobactam in Pseudomonas aeruginosa endotracheal tube biofilm.Emerg Microbes Infect. 2024 Dec;13(1):2420737. doi: 10.1080/22221751.2024.2420737. Epub 2024 Nov 17. Emerg Microbes Infect. 2024. PMID: 39530158 Free PMC article.
References
-
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. - DOI - PMC - PubMed
-
- Torres A, Niederman MS, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT) Eur Respir J. 2017;50(3):1700582. doi: 10.1183/13993003.00582-2017. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

