Electrostatic anti-CD33-antibody-protamine nanocarriers as platform for a targeted treatment of acute myeloid leukemia
- PMID: 36457063
- PMCID: PMC9716776
- DOI: 10.1186/s13045-022-01390-5
Electrostatic anti-CD33-antibody-protamine nanocarriers as platform for a targeted treatment of acute myeloid leukemia
Abstract
Background: Acute myeloid leukemia (AML) is a fatal clonal hematopoietic malignancy, which results from the accumulation of several genetic aberrations in myeloid progenitor cells, with a worldwide 5-year survival prognosis of about 30%. Therefore, the development of more effective therapeutics with novel mode of action is urgently demanded. One common mutated gene in the AML is the DNA-methyltransferase DNMT3A whose function in the development and maintenance of AML is still unclear. To specifically target "undruggable" oncogenes, we initially invented an RNAi-based targeted therapy option that uses the internalization capacity of a colorectal cancer specific anti-EGFR-antibody bound to cationic protamine and the anionic siRNA. Here, we present a new experimental platform technology of molecular oncogene targeting in AML.
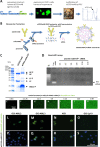
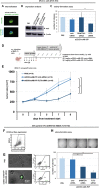
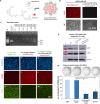
Methods: Our AML-targeting system consists of an internalizing anti-CD33-antibody-protamine conjugate, which together with anionic molecules such as siRNA or ibrutinib-Cy3.5 and cationic free protamine spontaneously assembles into vesicular nanocarriers in aqueous solution. These nanocarriers were analyzed concerning their physical properties and relevant characteristics in vitro in cell lines and in vivo in xenograft tumor models and patient-derived xenograft leukemia models with the aim to prepare them for translation into clinical application.
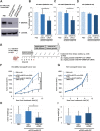
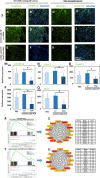
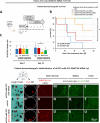
Results: The nanocarriers formed depend on a balanced electrostatic combination of the positively charged cationic protamine-conjugated anti-CD33 antibody, unbound cationic protamine and the anionic cargo. This nanocarrier transports its cargo safely into the AML target cells and has therapeutic activity against AML in vitro and in vivo. siRNAs directed specifically against two common mutated genes in the AML, the DNA-methyltransferase DNMT3A and FLT3-ITD lead to a reduction of clonal growth in vitro in AML cell lines and inhibit tumor growth in vivo in xenotransplanted cell lines. Moreover, oncogene knockdown of DNMT3A leads to increased survival of mice carrying leukemia patient-derived xenografts. Furthermore, an anionic derivative of the approved Bruton's kinase (BTK) inhibitor ibrutinib, ibrutinib-Cy3.5, is also transported by this nanocarrier into AML cells and decreases colony formation.
Conclusions: We report important results toward innovative personalized, targeted treatment options via electrostatic nanocarrier therapy in AML.
Keywords: DNMT3A inhibition; Gemtuzumab; Ibrutinib; Molecular targeted therapy; RNA interference.
© 2022. The Author(s).
Conflict of interest statement
NB, LW, AF, GL, WEB, SB have filed patent applications on electrostatic nanocarrier technology. All other authors disclose no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

