Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1
- PMID: 36457143
- PMCID: PMC10952572
- DOI: 10.1111/bph.15996
Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1
Abstract
Background and purpose: The protein PIEZO1 forms mechanically activated, calcium-permeable, non-selective cation channels in numerous cell types from several species. Options for pharmacological modulation are limited and so we modified a small-molecule agonist at PIEZO1 channels (Yoda1) to increase the ability to modulate these channels.
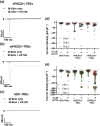
Experimental approach: Medicinal chemistry generated Yoda1 analogues that were tested in intracellular calcium and patch-clamp assays on cultured cells exogenously expressing human or mouse PIEZO1 or mouse PIEZO2. Physicochemical assays and wire myography assays on veins from mice with genetic disruption of PIEZO1.
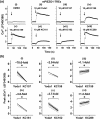
Key results: A Yoda1 analogue (KC159) containing 4-benzoic acid instead of the pyrazine of Yoda1 and its potassium salt (KC289) have equivalent or improved reliability, efficacy and potency, compared with Yoda1 in functional assays. Tested against overexpressed mouse PIEZO1 in calcium assays, the order of potency (as EC50 values, nM) was KC289, 150 > KC159 280 > Yoda1, 600). These compounds were selective for PIEZO1 over other membrane proteins, and the physicochemical properties were more suited to physiological conditions than those of Yoda1. The vasorelaxant effects were consistent with PIEZO1 agonism. In contrast, substitution with 2-benzoic acid failed to generate a modulator.
Conclusion and implications: 4-Benzoic acid modification of Yoda1 improves PIEZO1 agonist activity at PIEZO1 channels. We suggest naming this new modulator Yoda2. It should be a useful tool compound in physiological assays and facilitate efforts to identify a binding site. Such compounds may have therapeutic potential, for example, in diseases linked genetically to PIEZO1 such as lymphatic dysplasia.
Keywords: calcium channel; endothelial cell; mechanical force; medicinal chemistry; non-selective cation channel; pharmacology; vascular biology.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Automated patch‐clamp studies were performed at Nanion Technologies GmbH, which has interest in the commercial success of the SyncroPatch 384. Authors at Leeds and Homburg have interest in successful outcomes from research grants and studentships as indicated in the Acknowledgements. No other conflicts of interest are disclosed.
Figures








References
-
- Akbulut, Y. , Gaunt, H. J. , Muraki, K. , Ludlow, M. J. , Amer, M. S. , Bruns, A. , Vasudev, N. S. , Radtke, L. , Willot, M. , Hahn, S. , Seitz, T. , Ziegler, S. , Christmann, M. , Beech, D. J. , & Waldmann, H. (2015). (−)‐Englerin A is a potent and selective activator of TRPC4 and TRPC5 calcium channels. Angewandte Chemie (International Ed. in English), 54, 3787–3791. 10.1002/anie.201411511 - DOI - PMC - PubMed
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(S1), S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
-
- Alexander, S. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Amarosi, L. , Anderson, C. M. H. , Beart, P. M. , Broer, S. , Dawson, P. A. , Hagenbuch, B. , Hammond, J. R. , Inui, K.‐I. , … Verri, T. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Transporters. British Journal of Pharmacology, 178(S1), S412–S513. 10.1111/bph.15543 - DOI - PubMed
-
- Alexander, S. P. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Aldrich, R. W. , Attali, B. , Baggetta, A. M. , Becirovic, E. , Biel, M. , Bill, R. M. , Catterall, W. A. , … Zhu, M. (2021). The Concise Guide to PHARMACOLOGY 2021/22: Ion channels. British Journal of Pharmacology, 178(Suppl 1), S157–S245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

